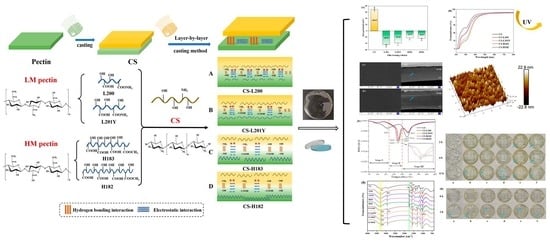
Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mw and Zeta Potentials of CS and Pectin
2.3. Preparation of Films
2.4. Characterization of Films
2.4.1. Measurement of Thickness and Mechanical Properties
2.4.2. Color
2.4.3. Optical Properties
2.4.4. Microstructure
2.4.5. Moisture Content (MC) and Water Solubility (WS)
2.4.6. Water Vapor Permeability (WVP)
2.4.7. Oxygen Permeability (OP) and Carbon Dioxide Permeability (CDP)
2.4.8. Thermogravimetric Analysis (TGA)
2.4.9. X-ray Diffraction (XRD)
2.4.10. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.11. Antifogging Properties
2.5. Statistical Analysis
3. Results and Discussion
3.1. Mw Distribution and Zeta Potential of CS and Pectin Film-Forming Solutions
3.2. Color and Opacity
3.3. Light Barrier Properties
3.4. Film Morphology
3.5. Moisture Content, Water Solubility, and Barrier Properties
3.6. Thickness and Mechanical Properties
3.7. Thermal Analysis
3.8. XRD
3.9. FT-IR Spectroscopy and the Mechanism of Forming Bilayer Films
3.10. Antifogging Property
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akhila, V.; Badwaik, L.S. Recent advancement in improvement of properties of polysaccharides and proteins based packaging film with added nanoparticles: A review. Int. J. Biol. Macromol. 2022, 203, 515–525. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Julian Mcclements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of Nanomaterials in Food Packaging and its Advanced Future Prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef] [PubMed]
- Abdalkarim, S.Y.H.; Chen, L.M.; Yu, H.Y.; Li, F.; Chen, X.; Zhou, Y.; Tam, K.C. Versatile nanocellulose-based nanohybrids: A promising-new class for active packaging applications. Int. J. Biol. Macromol. 2021, 182, 1915–1930. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tu, Z.; Sha, X.; Hu, Y.; Chen, N.; Wang, H. Fabrication and performance evaluation of pectin-fish gelatin-resveratrol preservative films. Food Chem. 2021, 361, 129832. [Google Scholar] [CrossRef]
- Lin, D.; Ma, Y.; Qin, W.; Loy, D.A.; Chen, H.; Zhang, Q. The structure, properties and potential probiotic properties of starch-pectin blend: A review. Food Hydrocoll. 2022, 129, 107644. [Google Scholar] [CrossRef]
- Min, T.; Zhou, L.; Sun, X.; Du, H.; Bian, X.; Zhu, Z.; Wen, Y. Enzyme-responsive food packaging system based on pectin-coated poly (lactic acid) nanofiber films for controlled release of thymol. Food Res. Int. 2022, 157, 111256. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, P.; Zhan, Y.; Shi, X.; Lin, J.; Du, Y.; Li, X.; Deng, H. Pectin/lysozyme bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial application. Carbohydr. Polym. 2015, 117, 687–693. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, X.; Liu, X.; Wang, Z. Fabrication of Pickering emulsion based on particles combining pectin and zein: Effects of pectin methylation. Carbohydr. Polym. 2021, 256, 117515. [Google Scholar] [CrossRef]
- Gates, S.J.; Shukla, A. Layer-by-layer assembly of readily detachable chitosan and poly(acrylic acid) polyelectrolyte multilayer films. J. Polym. Sci., Part B: Polym. Phys. 2017, 55, 127–131. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Teng, A.; Zhang, K.; Ma, Y.; Duan, S.; Li, S.; Guo, Y. Using cellulose nanofibers to reinforce polysaccharide films: Blending vs layer-by-layer casting. Carbohydr. Polym. 2020, 227, 115264. [Google Scholar] [CrossRef] [PubMed]
- Moalla, S.; Ammar, I.; Fauconnier, M.L.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H. Development and characterization of chitosan films carrying Artemisia campestris antioxidants for potential use as active food packaging materials. Int. J. Biol. Macromol. 2021, 183, 254–266. [Google Scholar] [CrossRef]
- Zeng, J.; Ren, X.; Zhu, S.; Gao, Y. Fabrication and characterization of an economical active packaging film based on chitosan incorporated with pomegranate peel. Int. J. Biol. Macromol. 2021, 192, 1160–1168. [Google Scholar] [CrossRef]
- Xia, C.; Wang, W.; Wang, L.; Liu, H.; Xiao, J. Multilayer zein/gelatin films with tunable water barrier property and prolonged antioxidant activity. Food Packag. Shelf Life 2019, 19, 76–85. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Molecular interactions in self-assembled nano-structures of chitosan-sodium alginate based polyelectrolyte complexes. Int. J. Biol. Macromol. 2018, 114, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.W. Fabrication of chitosan-based functional nanocomposite films: Effect of quercetin-loaded chitosan nanoparticles. Food Hydrocoll. 2021, 121, 107065. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Alves, V.D.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Yu, D.; Regenstein, J.M.; Dong, J.; Chen, W.; Xia, W. Chitosan/zein bilayer films with one-way water barrier characteristic: Physical, structural and thermal properties. Int. J. Biol. Macromol. 2022, 200, 378–387. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Ding, J.; Lai, P.F.H.; Xia, Y.; Ai, L. Structural features and emulsifying stability of a highly branched arabinogalactan from immature peach (Prunus persica) exudates. Food Hydrocoll. 2020, 104, 105721. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Devaraju, S.; Rambabu, K.; Banat, F.; Mittal, V. Silver-sepiolite (Ag-Sep) hybrid reinforced active gelatin/date waste extract (DSWE) blend composite films for food packaging application. Food Chem. 2022, 369, 130983. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Hernández-Cocoletzi, H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, B.; Jin, J. Transparent, water-stable, cellulose nanofiber-based packaging film with a low oxygen permeability. Carbohydr. Polym. 2020, 249, 116823. [Google Scholar] [CrossRef]
- Su, D.; Li, P.; Quek, S.; Huang, Z.; Yuan, Y.; Li, G.; Shan, Y. Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chem. 2019, 286, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Ruiz, M.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; Mcclements, D.J. Impact of pectin properties on lipid digestion under simulated gastrointestinal conditions: Comparison of citrus and banana passion fruit (Passiflora tripartita var. mollissima) pectins. Food Hydrocoll. 2016, 52, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Han, X.; Sun, Y.; Wang, X. Effect of ethanol content on rheology of film-forming solutions and properties of zein/chitosan film. Int. J. Biol. Macromol. 2019, 134, 807–814. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Otoni, C.G.; Moura, M.R.D.; Mattoso, L.H.C. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high- and low-methyl pectin films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative analysis of blend and bilayer films based on chitosan and gelatin enriched with LAE (lauroyl arginate ethyl) with antimicrobial activity for food packaging applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Xie, F.; Ren, X.; Wu, H.; Zhang, H.; Wu, Y.; Song, Z.; Ai, L. Pectins of different resources influences cold storage properties of corn starch gels: Structure-property relationships. Food Hydrocoll. 2022, 124, 107287. [Google Scholar] [CrossRef]
- Dai, W.; Zhou, L.; Gu, S.; Wang, W.; Xu, Z.; Zhou, X.; Ding, Y. Preparation and characterization of chitosan films incorporating epigallocatechin gallate: Microstructure, physicochemical, and bioactive properties. Int. J. Biol. Macromol. 2022, 211, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yu, D.; Yu, Z.; Zhang, L.; Xia, W. Thermally-induced crosslinking altering the properties of chitosan films: Structure, physicochemical characteristics and antioxidant activity. Food Packag. Shelf Life 2022, 34, 100948. [Google Scholar] [CrossRef]
- Kurek, M.; Benbettaieb, N.; Ščetar, M.; Chaudy, E.; Elez-Garofulić, I.; Repajić, M.; Klepac, D.; Valić, S.; Debeaufort, F.; Galić, K. Novel functional chitosan and pectin bio-based packaging films with encapsulated Opuntia-ficus indica waste. Food Biosci. 2021, 41, 100980. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Cui, J.; Ren, W.; Zhao, C.; Gao, W.; Tian, G.; Bao, Y.; Lian, Y.; Zheng, J. The structure property relationships of acid- and alkali-extracted grapefruit peel pectin. Carbohydr. Polym. 2020, 229, 115524. [Google Scholar] [CrossRef]
- Elizarova, I.S.; Luckham, P.F. Layer-by-layer adsorption: Factors affecting the choice of substrates and polymers. Adv. Colloid Interface Sci. 2018, 262, 1–20. [Google Scholar] [CrossRef]
- Rufato, K.B.; Souza, P.R.; De Oliveira, A.C.; Berton, S.B.R.; Sabino, R.M.; Muniz, E.C.; Popat, K.C.; Radovanovic, E.; Kipper, M.J.; Martins, A.F. Antimicrobial and cytocompatible chitosan, N,N,N-trimethyl chitosan, and tanfloc-based polyelectrolyte multilayers on gellan gum films. Int. J. Biol. Macromol. 2021, 183, 727–742. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Qin, C.; Yang, G.; Zhu, C.; Wei, M. Characterization of edible film fabricated with HG-type hawthorn pectin gained using different extraction methods. Carbohydr. Polym. 2022, 285, 119270. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Liu, D.; Ding, T.; Ye, X. Effect of degradation methods on the structural properties of citrus pectin. LWT 2015, 61, 630–637. [Google Scholar] [CrossRef]
- Çavdaroğlu, E.; Büyüktaş, D.; Farris, S.; Yemenicioğlu, A. Novel edible films of pectins extracted from low-grade fruits and stalk wastes of sun-dried figs: Effects of pectin composition and molecular properties on film characteristics. Food Hydrocoll. 2022, 135, 108136. [Google Scholar] [CrossRef]
- Thi Nguyen, T.; Pham, B.-T.T.; Nhien Le, H.; Bach, L.G.; Thuc, C.N.H. Comparative characterization and release study of edible films of chitosan and natural extracts. Food Packag. Shelf Life 2022, 32, 100830. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Huang, H.; Liu, A.; Liu, H.; Abid, N.; Ming, L. Chitosan/zein films incorporated with essential oil nanoparticles and nanoemulsions: Similarities and differences. Int. J. Biol. Macromol. 2022, 208, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Zhang, L.; Wang, X.; Li, L. Effects of plasticizer type and concentration on rheological, physico-mechanical and structural properties of chitosan/zein film. Int. J. Biol. Macromol. 2020, 143, 334–340. [Google Scholar] [CrossRef]
- Bhat, V.G.; Narasagoudr, S.S.; Masti, S.P.; Chougale, R.B.; Vantamuri, A.B.; Kasai, D. Development and evaluation of Moringa extract incorporated Chitosan/Guar gum/Poly (vinyl alcohol) active films for food packaging applications. Int. J. Biol. Macromol. 2022, 200, 50–60. [Google Scholar] [CrossRef]
- Lamarra, J.; Rivero, S.; Pinotti, A. Nanocomposite bilayers based on poly(vinyl alcohol) and chitosan functionalized with gallic acid. Int. J. Biol. Macromol. 2020, 146, 811–820. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, D.; Yu, D.; Regenstein, J.M.; Jiang, Q.; Dong, J.; Chen, W.; Xia, W. Modulating physicochemical, antimicrobial and release properties of chitosan/zein bilayer films with curcumin/nisin-loaded pectin nanoparticles. Food Hydrocoll. 2022, 133, 107955. [Google Scholar] [CrossRef]
- Indumathi, M.P.; Saral Sarojini, K.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef]
- Cefola, M.; Renna, M.; Pace, B. Marketability of ready-to-eat cactus pear as affected by temperature and modified atmosphere. J. Food Sci. Technol. 2014, 51, 25–33. [Google Scholar] [CrossRef]
- Zhuang, C.; Jiang, Y.; Zhong, Y.; Zhao, Y.; Deng, Y.; Yue, J.; Wang, D.; Jiao, S.; Gao, H.; Chen, H.; et al. Development and characterization of nano-bilayer films composed of polyvinyl alcohol, chitosan and alginate. Food Control 2018, 86, 191–199. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Dranca, F.; Talón, E.; Vargas, M.; Oroian, M. Microwave vs. conventional extraction of pectin from Malus domestica ‘Fălticeni’ pomace and its potential use in hydrocolloid-based films. Food Hydrocoll. 2021, 121, 107026. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Qiu, W.; Chen, T.; Yang, Y.; Wang, W.; Zhang, H. Ultrasonic treatment at different pH values affects the macromolecular, structural, and rheological characteristics of citrus pectin. Food Chem. 2021, 341, 128216. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Thai T, L.A. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocoll. 2017, 71, 141–148. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, H.; Sun, Q. Preparation of crosslinked active bilayer film based on chitosan and alginate for regulating ascorbate-glutathione cycle of postharvest cherry tomato (Lycopersicon esculentum). Int. J. Biol. Macromol. 2019, 130, 584–594. [Google Scholar] [CrossRef]
- Gomes Neto, R.J.; Genevro, G.M.; Paulo, L.D.A.; Lopes, P.S.; De Moraes, M.A.; Beppu, M.M. Characterization and in vitro evaluation of chitosan/konjac glucomannan bilayer film as a wound dressing. Carbohydr. Polym. 2019, 212, 59–66. [Google Scholar] [CrossRef]
- Ye, Y.; Zeng, F.; Zhang, M.; Zheng, S.; Li, J.; Fei, P. Hydrophobic edible composite packaging membrane based on low-methoxyl pectin/chitosan: Effects of lotus leaf cutin. Food Packag. Shelf Life 2020, 26, 100529. [Google Scholar] [CrossRef]
- Chetouani, A.; Follain, N.; Marais, S.; Rihouey, C.; Elkolli, M.; Bounekhel, M.; Benachour, D.; Le Cerf, D. Physicochemical properties and biological activities of novel blend films using oxidized pectin/chitosan. Int. J. Biol. Macromol. 2017, 97, 348–356. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, R.; Wang, B.; Chen, K. Development and characterization of bilayer films based on pea starch/polylactic acid and use in the cherry tomatoes packaging. Carbohydr. Polym. 2019, 222, 114912. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Li, F.; Wang, X.; Man, J.; Li, J.; Zhang, C.; Peng, S. A biodegradable chitosan-based composite film reinforced by ramie fibre and lignin for food packaging. Carbohydr. Polym. 2022, 281, 119078. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.; Zhang, J.; Niu, B.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Nisar, T.; Wang, Z.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Ružić, J.; Kalagasidis Krušić, M.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible blend films of pectin and poly(ethylene glycol): Preparation and physico-chemical evaluation. Food Hydrocoll. 2018, 77, 494–501. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef]
- Li, C.; Li, J. Preparation of chitosan-ferulic acid conjugate: Structure characterization and in the application of pharmaceuticals. Int. J. Biol. Macromol. 2017, 105, 1539–1543. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, L.; Chen, C.; Xie, J. Preparation and characterization of polyethylene antifogging film and its application in lettuce packaging. Food Control 2022, 139, 109075. [Google Scholar] [CrossRef]
- Rodrigues, M.Á.V.; Marangon, C.A.; Martins, V.D.C.A.; Plepis, A.M.D.G. Chitosan/gelatin films with jatobá resin: Control of properties by vegetal resin inclusion and degree of acetylation modification. Int. J. Biol. Macromol. 2021, 182, 1737–1745. [Google Scholar] [CrossRef]
- Sood, A.; Saini, C.S. Red pomelo peel pectin based edible composite films: Effect of pectin incorporation on mechanical, structural, morphological and thermal properties of composite films. Food Hydrocoll. 2022, 123, 107135. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, B.D.; Goh, K.L.; Muthoka, R.M.; Kim, J. Modulation of interfacial interactions toward strong and tough cellulose nanofiber-based transparent thin films with antifogging feature. Carbohydr. Polym. 2022, 278, 118974. [Google Scholar] [CrossRef]








| DE (%) | DA (%) | Mw (kDa) | Mn (kDa) | Mw/Mn | |
|---|---|---|---|---|---|
| CS | − | − | 619.5 | 514 | 1.21 |
| L200 | 26.9 | 21.9 | 87.46 | 16.4 | 5.33 |
| L201Y | 26.5 | 23.7 | 369.84 | 27.1 | 13.64 |
| H183 | 68.9 | − | 873.34 | 54.1 | 16.15 |
| H182 | 69.5 | − | 1243.64 | 53.3 | 23.31 |
| Film | Color | Opacity (mm–1) | ||||
|---|---|---|---|---|---|---|
| L | a | b | WI | |||
| CS | 37.79 ± 0.16 a | –0.36 ± 0.23 a | –0.45 ± 0.40 ab | 56.06 ± 0.14 e | 37.78 ± 0.16 a | 1.19 ± 0.07 a |
| L200 | 35.17 ± 0.62 cd | –0.40 ± 0.05 a | –0.33 ± 0.16 ab | 58.42 ± 0.61 abc | 35.16 ± 0.62 cd | 1.12 ± 0.10 a |
| L201Y | 34.42 ± 0.14 e | –0.31 ± 0.06 a | –0.01 ± 0.17 a | 59.16 ± 0.14 a | 34.42 ± 0.14 e | 1.12 ± 0.03 a |
| H183 | 34.96 ± 0.43 de | –0.38 ± 0.07 a | –0.48 ± 0.05 ab | 58.63 ± 0.43 ab | 34.96 ± 0.43 de | 1.14 ± 0.42 a |
| H182 | 34.65 ± 0.18 de | –0.30 ± 0.09 a | –1.11 ± 1.10 b | 58.98 ± 0.12 ab | 34.63 ± 0.16 de | 1.16 ± 0.06 a |
| CS-L200 | 35.73 ± 0.00 bc | –0.45 ± 0.01 a | –0.33 ± 0.04 ab | 57.86 ± 0.01 cd | 35.73 ± 0.00 bc | 1.03 ± 0.05 a |
| CS-L201Y | 35.98 ± 0.15 b | –0.46 ± 0.03 a | –0.35 ± 0.12 ab | 57.61 ± 0.15 d | 35.98 ± 0.15 b | 1.01 ± 0.03 a |
| CS-H183 | 35.35 ± 0.25 bcd | –0.40 ± 0.03 a | –0.23 ± 0.30 a | 58.23 ± 0.24 bcd | 35.35 ± 0.25 bcd | 0.99 ± 0.04 a |
| CS-H182 | 34.94 ± 0.76 de | –0.38 ± 0.07 a | –0.16 ± 0.02 a | 58.64 ± 0.76 ab | 34.94 ± 0.76 de | 0.97 ± 0.04 a |
| Film | MC (%) | WS (%) | WVP × 1010 (g·m–1·s–1·Pa–1) | OP × 109 (g·m–1·s–1) | CDP × 109 (g·m–1·s–1) | CDP/OP |
|---|---|---|---|---|---|---|
| CS | 19.81 ± 0.85 a | 13.73 ± 0.13 c | 1.46 ± 0.11 a | 1.39 ± 0.02 a | 2.75 ± 0.34 abc | 1.98 |
| L200 | 10.95 ± 0.07 b | 74.70 ± 7.91 a | 0.99 ± 0.07 d | 1.14 ± 0.12 ab | 3.04 ± 0.59 ab | 2.67 |
| L201Y | 12.50 ± 2.21 b | 74.89 ± 1.47 a | 0.99 ± 0.09 d | 1.00 ± 0.22 bc | 3.12 ± 0.30 a | 3.12 |
| H183 | 11.30 ± 0.44 b | 73.41 ± 3.64 a | 1.06 ± 0.11 d | 0.96 ± 0.35 bc | 2.99 ± 0.52 ab | 3.11 |
| H182 | 12.89 ± 4.64 b | 75.88 ± 2.27 a | 1.10 ± 0.05 cd | 0.95 ± 0.20 bc | 2.94 ± 0.13 ab | 3.09 |
| CS-L200 | 12.46 ± 0.12 b | 39.67 ± 0.85 b | 1.16 ± 0.05 bcd | 0.75 ± 0.09 c | 2.24 ± 0.35 c | 2.99 |
| CS-L201Y | 13.38 ± 2.64 b | 40.11 ± 1.15 b | 1.12 ± 0.07 bcd | 0.73 ± 0.07 c | 2.22 ± 0.15 c | 3.04 |
| CS-H183 | 12.20 ± 0.68 b | 40.22 ± 1.72 b | 1.24 ± 0.10 bc | 0.71 ± 0.05 c | 2.38 ± 0.35 bc | 3.35 |
| CS-H182 | 13.66 ± 2.30 b | 41.65 ± 0.90 b | 1.28 ± 0.14 b | 0.70 ± 0.07 c | 2.40 ± 0.20 bc | 3.43 |
| Film | Film Thickness (mm) | TS (MPa) | EAB (%) |
|---|---|---|---|
| CS | 0.082 ± 0.002 a | 49.15 ± 6.14 b | 4.70 ± 0.48 b |
| L200 | 0.062 ± 0.004 e | 21.15 ± 2.68 d | 2.59 ± 0.74 c |
| L201Y | 0.063 ± 0.004 de | 23.86 ± 3.42 cd | 3.88 ± 0.57 bc |
| H183 | 0.065 ± 0.003 cde | 24.64 ± 2.38 cd | 3.96 ± 1.19 bc |
| H182 | 0.070 ± 0.002 c | 30.61 ± 8.62 c | 3.93 ± 1.58 bc |
| CS-L200 | 0.065 ± 0.005 cde | 47.62 ± 0.50 b | 3.22 ± 0.58 bc |
| CS-L201Y | 0.066 ± 0.001 cde | 50.89 ± 1.74 b | 4.55 ± 0.47 b |
| CS-H183 | 0.067 ± 0.003 cd | 68.46 ± 6.16 a | 6.31 ± 0.12 a |
| CS-H182 | 0.075 ± 0.002 b | 71.49 ± 1.89 a | 6.67 ± 1.18 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Chang, X.; Ding, Z.; Xu, H.; Kong, H.; Chen, F.; Wang, R.; Shan, Y.; Ding, S. Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin. Foods 2022, 11, 3536. https://doi.org/10.3390/foods11213536
Fu X, Chang X, Ding Z, Xu H, Kong H, Chen F, Wang R, Shan Y, Ding S. Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin. Foods. 2022; 11(21):3536. https://doi.org/10.3390/foods11213536
Chicago/Turabian StyleFu, Xincheng, Xia Chang, Zemin Ding, Haishan Xu, Hui Kong, Fei Chen, Rongrong Wang, Yang Shan, and Shenghua Ding. 2022. "Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin" Foods 11, no. 21: 3536. https://doi.org/10.3390/foods11213536
APA StyleFu, X., Chang, X., Ding, Z., Xu, H., Kong, H., Chen, F., Wang, R., Shan, Y., & Ding, S. (2022). Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin. Foods, 11(21), 3536. https://doi.org/10.3390/foods11213536