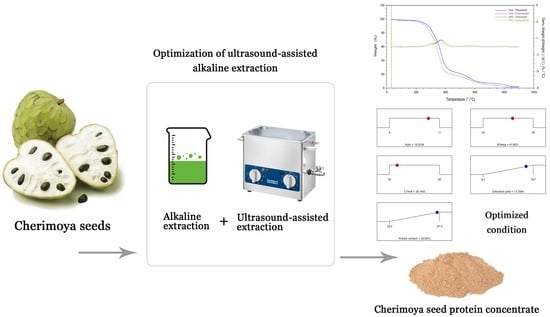
Extraction Optimization, Functional and Thermal Properties of Protein from Cherimoya Seed as an Unexploited By-Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ultrasound-Assisted Alkaline Extraction of Protein from Cherimoya Seeds
2.2. Conventional Alkaline Extraction
2.3. Optimization of the Ultrasound-Assisted Alkaline-Extraction Process
2.4. Thermal Stability
2.5. Techno-Functional Properties
2.5.1. Water-Holding Capacity
2.5.2. Oil-Binding Capacity
2.5.3. Foaming Capacity and Stability
3. Results and Discussion
3.1. Model Fitting and Statistical Analysis
3.2. Influence of Process Variables on Responses
3.3. Optimized Extraction Conditions
3.4. Thermal Properties of Cherimoya-Seed Proteins
3.5. Techno-Functional Properties of Cherimoya-Seed Proteins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perrone, A.; Yousefi, S.; Salami, A.; Papini, A.; Martinelli, F. Botanical, Genetic, Phytochemical and Pharmaceutical Aspects of Annona cherimola Mill. Sci. Hortic. 2022, 296, 110896. [Google Scholar] [CrossRef]
- Al Kazman, B.S.M.; Harnett, J.E.; Hanrahan, J.R. Traditional Uses, Phytochemistry and Pharmacological Activities of Annonacae. Molecules 2022, 27, 3462. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Gentile, C.; Porcu, A.; Agliassa, C.; Caradonna, F.; Bertea, C.M. Chemical Profile and Biological Activity of Cherimoya (Annona cherimola Mill.) and Atemoya (Annona atemoya) Leaves. Molecules 2020, 25, 2612. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.G.; Gutiérrez, M.T.; Mejías, F.J.R.; Molinillo, J.M.G.; Macías, F.A. An Overview of the Chemical Characteristics, Bioactivity and Achievements Regarding the Therapeutic Usage of Acetogenins from Annona cherimola Mill. Molecules 2021, 26, 2926. [Google Scholar] [CrossRef] [PubMed]
- Fuel, M.; Mesas, C.; Martínez, R.; Ortiz, R.; Quiñonero, F.; Prados, J.; Porres, J.M.; Melguizo, C. Antioxidant and antiproliferative potential of ethanolic extracts from Moringa oleifera, Tropaeolum tuberosum and Annona cherimola in colorrectal cancer cells. Biomed. Pharmacother. 2021, 143, 112248. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Boostani, S.; Jafari, S.M. Pea Proteins as Emerging Biopolymers for the Emulsification and Encapsulation of Food Bioactives. Food Hydrocoll. 2022, 126, 107474. [Google Scholar] [CrossRef]
- Avelar, Z.; Vicente, A.A.; Saraiva, J.A.; Rodrigues, R.M. The Role of Emergent Processing Technologies in Tailoring Plant Protein Functionality: New Insights. Trends Food Sci. Technol. 2021, 113, 219–231. [Google Scholar] [CrossRef]
- Majidiyan, N.; Hadidi, M.; Azadikhah, D.; Moreno, A. Protein Complex Nanoparticles Reinforced with Industrial Hemp Essential Oil: Characterization and Application for Shelf-Life Extension of Rainbow Trout Fillets. Food Chem. X 2022, 13, 100202. [Google Scholar] [CrossRef]
- Choe, U.; Chang, L.; Ohm, J.-B.; Chen, B.; Rao, J. Structure Modification, Functionality and Interfacial Properties of Kidney Bean (Phaseolus vulgaris L.) Protein Concentrate as Affected by Post-Extraction Treatments. Food Hydrocoll. 2022, 133, 108000. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Conde, J.; Pagan, J. Optimisation of steam blanching on enzymatic activity, color and protein degradation of alfalfa (Medicago sativa) to improve some quality characteristics of its edible protein. Food Chem. 2019, 276, 591–598. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, P.; Wani, S.A. Effect of Extraction Parameters on the Isolation of Fenugreek Seed Protein and Characterization of Fenugreek Protein Concentrate. Qual. Assur. Saf. Crops Foods 2022, 14, 12–23. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, B.; Morshedi, A.; Akbarian, M.; Akbarian, A.; Hadidi, M.; Moayedi, F. Effect of High Pressure Processing of Food Characteristics: A Review of Quality Aspect. Int. J. Biosci. (IJB) 2014, 4, 193–205. [Google Scholar]
- Hashemi Moosavi, M.; Mousavi Khaneghah, A.; Javanmardi, F.; Hadidi, M.; Hadian, Z.; Jafarzadeh, S.; Huseyn, E.; Sant’Ana, A.S. A Review of Recent Advances in the Decontamination of Mycotoxin and Inactivation of Fungi by Ultrasound. Ultrason. Sonochem. 2021, 79, 105755. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted Extraction and Modification of Plant-based Proteins: Impact on Physicochemical, Functional, and Nutritional Properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pouramin, S. Optimization of Extraction and Deamidation of Edible Protein from Evening Primrose (Oenothera biennis L.) Oil Processing by-Products and Its Effect on Structural and Techno-Functional Properties. Food Chem. 2021, 334, 127613. [Google Scholar] [CrossRef]
- Hadidi, M.; Khaksar, F.B.; Pagan, J.; Ibarz, A. Application of Ultrasound-Ultrafiltration-Assisted Alkaline Isoelectric Precipitation (UUAAIP) Technique for Producing Alfalfa Protein Isolate for Human Consumption: Optimization, Comparison, Physicochemical, and Functional Properties. Food Res. Int. 2020, 130, 108907. [Google Scholar] [CrossRef]
- AOAC Official Method of Analysis (2005) (AOAC 992-23). Available online: https://vdocument.in/aoac-official-method-of-analysis-2005-aoac-992-23.html?page=2 (accessed on 6 November 2022).
- Hadidi, M.; Amoli, P.I.; Jelyani, A.Z.; Hasiri, Z.; Rouhafza, A.; Ibarz, A.; Khaksar, F.B.; Tabrizi, S.T. Polysaccharides from Pineapple Core as a Canning By-Product: Extraction Optimization, Chemical Structure, Antioxidant and Functional Properties. Int. J. Biol. Macromol. 2020, 163, 2357–2364. [Google Scholar] [CrossRef]
- Raihana, A.R.N.; Marikkar, J.M.N.; Amin, I.; Shuhaimi, M. A Review on Food Values of Selected Tropical Fruits’ Seeds. Int. J. Food Prop. 2015, 18, 2380–2392. [Google Scholar] [CrossRef]
- Gueffai, A.; Gonzalez-Serrano, D.J.; Christodoulou, M.C.; Orellana-Palacios, J.C.; Ortega, M.L.S.; Ouldmoumna, A.; Kiari, F.Z.; Ioannou, G.D.; Kapnissi-Christodoulou, C.P.; Moreno, A.; et al. Phenolics from Defatted Black Cumin Seeds (Nigella sativa L.): Ultrasound-Assisted Extraction Optimization, Comparison, and Antioxidant Activity. Biomolecules 2022, 12, 1311. [Google Scholar] [CrossRef]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.-B.; Chen, B.; Rao, J. Effect of Alkaline Extraction PH on Structure Properties, Solubility, and Beany Flavor of Yellow Pea Protein Isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fu, Q. Optimization of Ultrasound-Assisted Extraction Process of Perilla Seed Meal Proteins. Food Sci. Biotechnol. 2012, 21, 1701–1706. [Google Scholar] [CrossRef]
- Eromosele, C.O.; Arogundade, L.A.; Eromosele, I.C.; Ademuyiwa, O. Extractability of African Yam Bean (Sphenostylis stenocarpa) Protein in Acid, Salt and Alkaline Aqueous Media. Food Hydrocoll. 2008, 22, 1622–1628. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of Ultrasound-Assisted Extraction (UAE) Process for the Recovery of Bioactive Compounds from Bitter Gourd Using Response Surface Methodology (RSM). Food Bioprod. Process. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Yao, H.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Advances in Renewable Plant-Derived Protein Source: The Structure, Physicochemical Properties Affected by Ultrasonication. Ultrason. Sonochem. 2019, 53, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.-B.; Chen, B.; Rao, J. Plant Proteins from Green Pea and Chickpea: Extraction, Fractionation, Structural Characterization and Functional Properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Ganesan, K.; Selvaraj, K.; Subba Rao, P.V. Studies on the Functional Properties of Protein Concentrate of Kappaphycus alvarezii (Doty) Doty—An Edible Seaweed. Food Chem. 2014, 153, 353–360. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, T.; Hong, X.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Modification of Functional Properties of Perilla Protein Isolate by High-Intensity Ultrasonic Treatment and the Stability of o/w Emulsion. Food Chem. 2022, 368, 130848. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Reetu; Punia, S.; Dhakane-Lad, J.; Singh, S.; Dhumal, S.; Chandra Pradhan, P.; Bhushan, B.; et al. Functional Characterization of Plant-Based Protein to Determine Its Quality for Food Applications. Food Hydrocoll. 2022, 123, 106986. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, Molecular and Thermal Properties of High-Intensity Ultrasound (HIUS) Treated Protein Isolates from Album (Chenopodium album) Seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High Intensity Ultrasound Treatment of Protein Isolate Extracted from Dephenolized Sunflower Meal: Effect on Physicochemical and Functional Properties. Ultrason. Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Daba, S.D.; Morris, C.F. Pea Proteins: Variation, Composition, Genetics, and Functional Properties. Cereal Chem. 2022, 99, 8–20. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, K.; Bai, Y.; Li, B.; Xu, W. Effect of High Intensity Ultrasound on Physicochemical, Interfacial and Gel Properties of Chickpea Protein Isolate. LWT 2020, 129, 109563. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Bamdad, F.; Wang, Y.; Tian, Z.; Temelli, F.; Han, J.; Chen, L. Optimization of Lentil Protein Extraction and the Influence of Process PH on Protein Structure and Functionality. LWT Food Sci. Technol. 2014, 57, 461–469. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of High Intensity Ultrasound on Structure and Foaming Properties of Pea Protein Isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]




| Factors | Responses | ||||
|---|---|---|---|---|---|
| Run | A: pH | B: Temperature °C | C: Time min | Extraction Yield % | Protein Content % |
| 1 | 9 (−1) | 50 (+1) | 40 (0) | 11.2 | 37.1 |
| 2 | 9 (−1) | 40 (0) | 20 (−1) | 12.4 | 41.3 |
| 3 | 11 (+1) | 40 (0) | 20 (−1) | 17.1 | 63.2 |
| 4 | 10 (0) | 40 (0) | 40 (0) | 15.3 | 64.5 |
| 5 | 10 (0) | 30 (−1) | 60 (+1) | 10.5 | 46.9 |
| 6 | 10 (0) | 30 (−1) | 20 (−1) | 11.6 | 44.1 |
| 7 | 9 (−1) | 30 (−1) | 40 (0) | 9.1 | 35.8 |
| 8 | 10 (0) | 40 (0) | 40 (0) | 15.5 | 65.2 |
| 9 | 10 (0) | 40 (0) | 40 (0) | 15 | 66.8 |
| 10 | 10 (0) | 50 (+1) | 60 (+1) | 10.4 | 28.3 |
| 11 | 11 (+1) | 40 (0) | 60 (+1) | 12.8 | 32.7 |
| 12 | 10 (0) | 50 (+1) | 20 (−1) | 18.7 | 47.5 |
| 13 | 11 (+1) | 30 (−1) | 40 (0) | 14.7 | 58.1 |
| 14 | 11 (+1) | 50 (+1) | 40 (0) | 15.3 | 36.9 |
| 15 | 10 (0) | 40 (0) | 40 (0) | 14.2 | 67.4 |
| 16 | 9 (−1) | 40 (0) | 60 (+1) | 9.1 | 31.8 |
| Extraction Yield | Protein Content | |||||
|---|---|---|---|---|---|---|
| Source | Coefficient Estimate | F-Value | p-Value | Coefficient Estimate | F-Value | p-Value |
| Model | 15.00 | 20.15 | 0.0008 | 65.98 | 24.72 | 0.0005 |
| A-pH | 2.26 | 62.88 | 0.0002 | 5.61 | 19.40 | 0.0045 |
| B-Temp | 1.21 | 18.06 | 0.0054 | −4.39 | 11.86 | 0.0137 |
| C-Time | −2.13 | 55.47 | 0.0003 | −7.05 | 30.61 | 0.0015 |
| AB | −0.3750 | 0.8637 | 0.3886 | −5.62 | 9.74 | 0.0205 |
| AC | −0.2500 | 0.3839 | 0.5583 | −5.25 | 8.49 | 0.0269 |
| BC | −1.80 | 19.90 | 0.0043 | −5.50 | 9.32 | 0.0224 |
| A2 | −1.19 | 8.66 | 0.0258 | −11.72 | 42.34 | 0.0006 |
| B2 | −1.24 | 9.41 | 0.0220 | −12.27 | 46.40 | 0.0005 |
| C2 | −0.9625 | 5.69 | 0.0544 | −12.00 | 44.35 | 0.0006 |
| Lack of Fit | 2.99 | 0.1964 | 13.20 | 0.0310 | ||
| R2 | 0.9680 | 0.9737 | ||||
| Adj-R2 | 0.9299 | 0.9344 | ||||
| C.V. (%) | 6.06 | 7.51 | ||||
| Extraction Technique | ||
|---|---|---|
| Parameters | Conventional | Ultrasound-Assisted |
| Extraction yield (g/100 g) | 12.2 ± 1.3 b | 18.3 ± 1.7 a |
| Protein content (g/100 g) | 54.5 ± 4.6 b | 66.1 ± 3.1 a |
| Water-holding capacity (g water/g) | 3.34 ± 0.31 b | 3.90 ± 0.17 a |
| Oil-binding capacity (g oil/g) | 3.37 ± 0.19 c | 4.11 ± 0.09 a |
| Foam capacity (%) | 165.8 ± 14.6 b | 192.5 ± 9.9 a |
| Foam stability (%) | 53.6 ± 5.9 a | 52.8 ± 4.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orellana-Palacios, J.C.; Hadidi, M.; Boudechiche, M.Y.; Ortega, M.L.S.; Gonzalez-Serrano, D.J.; Moreno, A.; Kowalczewski, P.Ł.; Bordiga, M.; Mousavi Khanegah, A. Extraction Optimization, Functional and Thermal Properties of Protein from Cherimoya Seed as an Unexploited By-Product. Foods 2022, 11, 3694. https://doi.org/10.3390/foods11223694
Orellana-Palacios JC, Hadidi M, Boudechiche MY, Ortega MLS, Gonzalez-Serrano DJ, Moreno A, Kowalczewski PŁ, Bordiga M, Mousavi Khanegah A. Extraction Optimization, Functional and Thermal Properties of Protein from Cherimoya Seed as an Unexploited By-Product. Foods. 2022; 11(22):3694. https://doi.org/10.3390/foods11223694
Chicago/Turabian StyleOrellana-Palacios, Jose C., Milad Hadidi, Marwa Yassamine Boudechiche, Maria Lopez S. Ortega, Diego J. Gonzalez-Serrano, Andres Moreno, Przemysław Łukasz Kowalczewski, Matteo Bordiga, and Amin Mousavi Khanegah. 2022. "Extraction Optimization, Functional and Thermal Properties of Protein from Cherimoya Seed as an Unexploited By-Product" Foods 11, no. 22: 3694. https://doi.org/10.3390/foods11223694
APA StyleOrellana-Palacios, J. C., Hadidi, M., Boudechiche, M. Y., Ortega, M. L. S., Gonzalez-Serrano, D. J., Moreno, A., Kowalczewski, P. Ł., Bordiga, M., & Mousavi Khanegah, A. (2022). Extraction Optimization, Functional and Thermal Properties of Protein from Cherimoya Seed as an Unexploited By-Product. Foods, 11(22), 3694. https://doi.org/10.3390/foods11223694