The Discrimination and Characterization of Volatile Organic Compounds in Different Areas of Zanthoxylum bungeanum Pericarps and Leaves by HS-GC-IMS and HS-SPME-GC-MS
Abstract
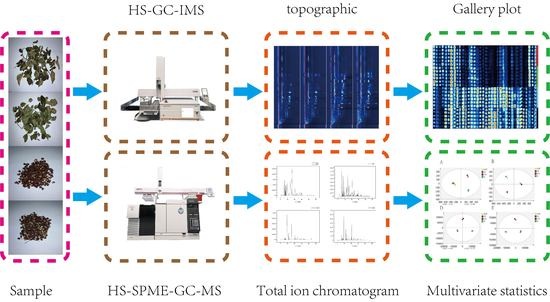
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. HS-GC-IMS Analysis
2.3. SPME Optimization
2.4. Conditions of GC-MS
2.5. Statistical Analysis
3. Results and Discussion
3.1. HS-GC-IMS Topographic Plots in SHJY, SHJ, SJY and SJ
3.2. Fingerprint Analysis of VOCs in SHJ, SJ, SHJY, SJY
3.3. Component Content Analysis of HS-SPME-GC-MS Results
3.4. Multivariate Statistical Analysis
3.5. Comprehensive Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Liu, J.; Shen, P.; Cao, Y.; Lu, X.; Gao, X.; Fu, Y.; Liu, B.; Zhang, N. Zanthoxylum bungeanum pericarp extract prevents dextran sulfate sodium-induced experimental colitis in mice via the regulation of TLR4 and TLR4-related signaling pathways. Int. Immunopharmacol. 2016, 41, 127–135. [Google Scholar] [CrossRef]
- Jing, N.; Wang, M.; Gao, M.; Zhong, Z.; Ma, Y.; Wei, A. Color sensory characteristics, nutritional components and antioxidant capacity of Zanthoxylum bungeanum Maxim. as affected by different drying methods. Ind. Crops Prod. 2021, 160, 113167. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.H.; Luo, B.; Sun, Y.N.; Kim, Y.H.; Wei, A.Z.; Gao, J. Isobutylhydroxyamides from Zanthoxylum bungeanum and Their Suppression of NO Production. Molecules 2016, 21, 1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; He, Z.; Zhang, D.; Chen, X.; Li, H. Effect of pepper (Zanthoxylum bungeanum Maxim.) essential oil on quality changes in rabbit meat patty during chilled storage. J. Food Sci. Technol. 2022, 59, 179–191. [Google Scholar] [CrossRef]
- He, F.; Li, D.; Wang, D.; Deng, M. Extraction and purification of quercitrin, hyperoside, rutin, and afzelin from Zanthoxylum bungeanum maxim leaves using an aqueous two-phase system. J. Food Sci. 2016, 81, C1593–C1602. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Hu, K.; Zeng, L.; Pan, Y.; Huang, H. Change in aromatic components of banana during the preparation process of juice and microcapsule powder. Int. J. Food Sci. Technol. 2011, 46, 1398–1405. [Google Scholar] [CrossRef]
- Shi, J.; Fei, X.; Hu, Y.; Liu, Y.; Wei, A. Identification of key genes in the synthesis pathway of volatile terpenoids in fruit of Zanthoxylum bungeanum maxim. Forests 2019, 10, 328. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Luo, Z.; Wang, D. Efficient quantification of the phenolic profiles of Zanthoxylum bungeanum leaves and correlation between chromatographic fingerprint and antioxidant activity. Nat. Prod. Res. 2015, 29, 2024–2029. [Google Scholar] [CrossRef]
- Chang, S.Y.; Xiao, K.; Zhang, J.Q.; Zhong, K.; Grosu, E.; Gao, Z.; Wu, Y.P.; Gao, H. Antibacterial and Antibiofilm Effects of Zanthoxylum bungeanum Leaves against Staphylococcus aureus. Nat. Prod. Commun. 2018, 13, 1001–1006. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Mesa, M.; Ropartz, D.; Garcia-Campana, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion mobility spectrometry in food analysis: Principles, current applications and future trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ríos, G.A.; Mirabelli, M.F. Solid phase microextraction-mass spectrometry: Metanoia. TrAC Trends Anal. Chem. 2019, 112, 201–211. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Wu, J.; Zou, X.; Ho, C.-T.; Liang, L.; Yan, X.; Zhou, X. Rapid qualitative and quantitative analysis of strong aroma base liquor based on SPME-MS combined with chemometrics. Food Sci. Hum. Wellness 2021, 10, 362–369. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.; Lin, R.; Li, X.; Ding, H.; Han, L.; Yang, W.; Song, X.; Li, W.; Qu, H.; et al. Application and development trends of gas chromatography–ion mobility spectrometry for traditional Chinese medicine, clinical, food and environmental analysis. Microchem. J. 2021, 168, 106527. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Yan, M.; Lin, X.; Chen, Y. Gas chromatographic-ion mobility spectrometry combined with a multivariate analysis model exploring the characteristic changes of odor components during the processing of black sesame. Anal Methods 2020, 12, 4987–4995. [Google Scholar] [CrossRef]
- Garcia-Nicolas, M.; Arroyo-Manzanares, N.; de Dios Hernandez, J.; Guillen, I.; Vizcaino, P.; Sanchez-Rubio, M.; Lopez-Garcia, I.; Hernandez-Cordoba, M.; Vinas, P. Ion mobility spectrometry and mass spectrometry coupled to gas chromatography for analysis of microbial contaminated cosmetic creams. Anal. Chim. Acta 2020, 1128, 52–61. [Google Scholar] [CrossRef]
- Xing, Y.; Yu, Z.; Hu, X.; Yin, J.; Fan, T.; Fu, Z.; Pan, G.; Liu, E.; Zhou, J.; Han, L. Characterization of volatile organic compounds in Polygonum multiflorum and two of its processed products based on multivariate statistical analysis for processing technology monitoring. J. Chromatogr. A 2022, 1680, 463431. [Google Scholar] [CrossRef] [PubMed]
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 703, 114–123. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Lv, W.; Lin, T.; Ren, Z.; Jiang, Y.; Zhang, J.; Bi, F.; Gu, L.; Hou, H.; He, J. Rapid discrimination of Citrus reticulata ‘Chachi’ by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef]
- Qi, H.; Ding, S.; Pan, Z.; Li, X.; Fu, F. Characteristic Volatile fingerprints and odor activity values in different citrus-tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules 2020, 25, 6027. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Wang, Y.; Wang, X.; Chen, F.; Wang, X. Characterization of aroma-impact compounds in dry jujubes (Ziziphus jujube Mill.) by aroma extract dilution analysis (AEDA) and gas chromatography-mass spectrometer (GC-MS). Int. J. Food Prop. 2018, 21, 1844–1853. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Quispe, C.; Islam, M.A.; Ali, E.S.; Saha, S.; Asha, U.H.; Mondal, M.; Razis, A.F.A.; Sunusi, U.; Kamal, R.M.; et al. Effects of nerol on paracetamol-induced liver damage in Wistar albino rats. Biomed. Pharmacother. 2021, 140, 111732. [Google Scholar] [CrossRef]
- An, P.; Yang, X.; Yu, J.; Qi, J.; Ren, X.; Kong, Q. α-terpineol and terpene-4-ol, the critical components of tea tree oil, exert antifungal activities in vitro and in vivo against Aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control 2019, 98, 42–53. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kumar, S. A-terpinyl acetate: A natural monoterpenoid from Elettaria cardamomum as multi-target directed ligand in Alzheimer’s disease. J. Funct. Foods 2020, 68, 103892. [Google Scholar] [CrossRef]
- Seo, E.; Shin, Y.K.; Hsieh, Y.S.; Lee, J.M.; Seol, G.H. Linalyl acetate as a potential preventive agent against muscle wasting in rheumatoid arthritis rats chronically exposed to nicotine. J. Pharmacol. Sci. 2021, 147, 27–32. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Characterization of Key Odorants in Hanyuan and Hancheng Fried Pepper (Zanthoxylum bungeanum) Oil. J. Agric Food Chem. 2020, 68, 6403–6411. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Seo, S.; Lamichhane, S.; Seo, J.; Hong, J.T.; Cha, H.J.; Yun, J. Limonene has anti-anxiety activity via adenosine A2A receptor-mediated regulation of dopaminergic and GABAergic neuronal function in the striatum. Phytomedicine 2021, 83, 153474. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Castro, M.A.; Crespo, R.; Galle, M.; Garcia de Bravo, M. Anti-cancer mechanisms of linalool and 1,8-cineole in non-small cell lung cancer A549 cells. Heliyon 2020, 6, e05639. [Google Scholar] [CrossRef]
- Ji, Y.; Li, S.; Ho, C.-T. Chemical composition, sensory properties and application of Sichuan pepper (Zanthoxylum genus). Food Sci. Hum. Wellness 2019, 8, 115–125. [Google Scholar] [CrossRef]
- Jaroque, G.N.; Sartorelli, P.; Caseli, L. The effect of the monocyclic monoterpene tertiary alcohol γ-terpineol on biointerfaces containing cholesterol. Chem. Phys. Lipids 2020, 230, 104915. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, J.; Griffin, S.; Valdramidis, V.P.; Camilleri, K.; Falzon, O. Principal component analysis of hyperspectral data for early detection of mould in cheeselets. Curr. Res. Food Sci. 2021, 4, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, S.; Xi, W.; Yang, J.; Liao, Q.; Lan, J.; Lv, Y.; Tang, J. Comparison of volatile components in fresh and dried Zanthoxylum bungeanum Maxim. Food Sci. Biotechnol. 2019, 28, 1083–1092. [Google Scholar] [CrossRef]





| Number | Compound | CAS | Formula | RI | Rt [s] | Dt [a.u.] |
|---|---|---|---|---|---|---|
| 1 | E-2-Hexenal monomer | 6728-26-3 | C6H10O | 840.4 | 338.459 | 1.18769 |
| 2 | E-2-Hexenal dimer | 6728-26-3 | C6H10O | 839 | 336.845 | 1.52614 |
| 3 | Methyl-5-hepten-2-one | 110-93-0 | C8H14O | 986.2 | 573.354 | 1.18323 |
| 4 | α-Terpinene monomer | 99-86-5 | C10H16 | 1018.9 | 634.386 | 1.22448 |
| 5 | α-Terpinene dimer | 99-86-5 | C10H16 | 1018.1 | 632.892 | 1.72976 |
| 6 | 1,8-Cineole monomer | 470-82-6 | C10H18O | 1033.2 | 661.043 | 1.29673 |
| 7 | 1,8-Cineole dimer | 470-82-6 | C10H18O | 1032.9 | 660.445 | 1.73863 |
| 8 | 2-Hexanol monomer | 626-93-7 | C6H14O | 784.9 | 274.17 | 1.28425 |
| 9 | 2-Hexanol dimer | 626-93-7 | C6H14O | 783.5 | 272.736 | 1.57117 |
| 10 | 2,3-Butanedione | 431-03-8 | C4H6O2 | 564.4 | 132.799 | 1.17274 |
| 11 | Linalool oxide monomer | 60047-17-8 | C10H18O2 | 1078.7 | 746.151 | 1.26752 |
| 12 | Linalool oxide dimer | 60047-17-8 | C10H18O2 | 1079.1 | 746.932 | 1.82662 |
| 13 | 2,3-Butanediol | 513-85-9 | C4H10O2 | 771.4 | 262.286 | 1.36835 |
| 14 | Acetic acid monomer | 64-19-7 | C2H4O2 | 578.8 | 139.508 | 1.04966 |
| 15 | Acetic acid dimer | 64-19-7 | C2H4O2 | 579.4 | 139.804 | 1.15007 |
| 16 | Pentanol monomer | 71-41-0 | C5H12O | 786 | 275.477 | 1.25575 |
| 17 | Pentanol dimer | 71-41-0 | C5H12O | 784.9 | 274.196 | 1.50928 |
| 18 | 2-Acetylfuran monomer | 1192-62-7 | C6H6O2 | 884.9 | 389.997 | 1.12002 |
| 19 | 2-Acetylfuran dimer | 1192-62-7 | C6H6O2 | 884.9 | 389.997 | 1.44292 |
| 20 | β-Ocimene monomer | 13877-91-3 | C10H16 | 1050.1 | 692.666 | 1.25509 |
| 21 | β-Ocimene dimer | 13877-91-3 | C10H16 | 1050 | 692.522 | 1.69652 |
| 22 | 4-Terpineol monomer | 562-74-3 | C10H18O | 1168.6 | 914.198 | 1.2266 |
| 23 | 4-Terpineol dimer | 562-74-3 | C10H18O | 1169.1 | 915.08 | 1.73235 |
| 24 | Nerol monomer | 106-25-2 | C10H18O | 1232.2 | 1033.082 | 1.2273 |
| 25 | Nerol dimer | 106-25-2 | C10H18O | 1228.9 | 1026.787 | 1.73416 |
| 26 | Phenylacetic acid | 103-82-2 | C8H8O2 | 1250.7 | 1067.554 | 1.32855 |
| 27 | Acetone | 67-64-1 | C3H6O | 529.6 | 116.559 | 1.1205 |
| 28 | Ethanol | 64-17-5 | C2H6O | 509.4 | 107.107 | 1.12947 |
| 29 | Ethyl acetate monomer | 141-78-6 | C4H8O2 | 603.3 | 150.951 | 1.09808 |
| 30 | Ethyl acetate dimer | 141-78-6 | C4H8O2 | 601.6 | 150.163 | 1.34211 |
| 31 | Furfural | 98-01-1 | C5H4O2 | 846.1 | 345.037 | 1.08274 |
| 32 | Neryl acetate | 141-12-8 | C12H20O2 | 1367.5 | 1285.92 | 1.22894 |
| 33 | δ-Octalactone | 698-76-0 | C8H14O2 | 1272.2 | 1107.751 | 1.28219 |
| 34 | Furaneol | 3658-77-3 | C6H8O3 | 1083.7 | 755.42 | 1.19676 |
| 35 | Diethyl butanedioate | 123-25-1 | C8H14O4 | 1193.7 | 961.041 | 1.29436 |
| 36 | Isopropyl acetate | 108-21-4 | C5H10O2 | 658.7 | 176.819 | 1.16357 |
| 37 | 2,5-Dimethylfuran | 625-86-5 | C6H8O | 690.4 | 192.347 | 1.35536 |
| 38 | 2-Methyl butanol | 137-32-6 | C5H12O | 740.3 | 235.425 | 1.24002 |
| 39 | (E)-2-Pentenal | 1576-87-0 | C5H8O | 790 | 280.073 | 1.10915 |
| 40 | Butanoic acid | 107-92-6 | C4H8O2 | 854.4 | 354.732 | 1.1635 |
| 41 | (E,E)-2,4-Hexadienal monomer | 142-83-6 | C6H8O | 909.9 | 431.414 | 1.11381 |
| 42 | (E,E)-2,4-Hexadienal dimer | 142-83-6 | C6H8O | 909.9 | 431.414 | 1.45388 |
| 43 | Pentanoic acid | 109-52-4 | C5H10O2 | 898.2 | 409.694 | 1.23289 |
| 44 | Dipropyl sulfide | 111-47-7 | C6H14S | 879.3 | 383.546 | 1.15847 |
| 45 | Dimethyl disulfide | 624-92-0 | C2H6S2 | 751.4 | 245.056 | 1.06575 |
| 46 | 2-Butoxyethanol | 111-76-2 | C6H14O2 | 898.4 | 410.086 | 1.20642 |
| 47 | α-Pinene monomer | 80-56-8 | C10H16 | 922.1 | 454.074 | 1.22494 |
| 48 | α-Pinene dimer | 80-56-8 | C10H16 | 922.3 | 454.5 | 1.67418 |
| 49 | 2-Cyclohexen-1-one | 930-68-7 | C6H8O | 941.1 | 489.49 | 1.11358 |
| 50 | β-Pinene monomer | 127-91-3 | C10H16 | 970.5 | 544.16 | 1.22525 |
| 51 | β-Pinene dimer | 127-91-3 | C10H16 | 970.2 | 543.605 | 1.64775 |
| 52 | β-Pinene tripolymer | 127-91-3 | C10H16 | 969 | 541.383 | 1.73717 |
| 53 | 4-Methylguaiacol | 93-51-6 | C8H10O2 | 1186.6 | 947.846 | 1.17166 |
| 54 | Carveol | 99-48-9 | C10H16O | 1232.9 | 1034.287 | 1.19266 |
| 55 | Allyldisulfide | 2179-57-9 | C6H10S2 | 1066 | 722.333 | 1.19546 |
| 56 | Pentanal monomer | 110-62-3 | C5H10O | 689.4 | 191.444 | 1.18336 |
| 57 | Pentanal dimer | 110-62-3 | C5H10O | 689.9 | 191.877 | 1.43071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yin, J.; Ding, H.; Li, W.; Han, L.; Yang, W.; Li, F.; Song, X.; Bie, S.; Gong, X.; et al. The Discrimination and Characterization of Volatile Organic Compounds in Different Areas of Zanthoxylum bungeanum Pericarps and Leaves by HS-GC-IMS and HS-SPME-GC-MS. Foods 2022, 11, 3745. https://doi.org/10.3390/foods11223745
Wu X, Yin J, Ding H, Li W, Han L, Yang W, Li F, Song X, Bie S, Gong X, et al. The Discrimination and Characterization of Volatile Organic Compounds in Different Areas of Zanthoxylum bungeanum Pericarps and Leaves by HS-GC-IMS and HS-SPME-GC-MS. Foods. 2022; 11(22):3745. https://doi.org/10.3390/foods11223745
Chicago/Turabian StyleWu, Xinlong, Jiaxin Yin, Hui Ding, Wei Li, Lifeng Han, Wenzhi Yang, Fangyi Li, Xinbo Song, Songtao Bie, Xingchu Gong, and et al. 2022. "The Discrimination and Characterization of Volatile Organic Compounds in Different Areas of Zanthoxylum bungeanum Pericarps and Leaves by HS-GC-IMS and HS-SPME-GC-MS" Foods 11, no. 22: 3745. https://doi.org/10.3390/foods11223745
APA StyleWu, X., Yin, J., Ding, H., Li, W., Han, L., Yang, W., Li, F., Song, X., Bie, S., Gong, X., Yu, H., & Li, Z. (2022). The Discrimination and Characterization of Volatile Organic Compounds in Different Areas of Zanthoxylum bungeanum Pericarps and Leaves by HS-GC-IMS and HS-SPME-GC-MS. Foods, 11(22), 3745. https://doi.org/10.3390/foods11223745