Effects of Hay, Baleage, and Soybean Hulls Waste Used as Supplemental Feeds on the Nutritional Profile of Grass-Finished Beef
Abstract
:1. Introduction
2. Materials and Methods
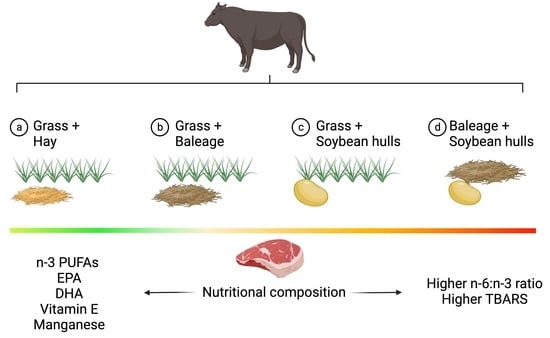
2.1. Experimental Design, Animals, and Diets
2.2. Sample Collection and Preparation
2.2.1. Feed Samples
2.2.2. Meat Samples
2.3. Feed Chemical Analysis
2.3.1. Proximate Analysis
2.3.2. Phytochemical Analysis
2.4. Fatty Acid Analysis of Feed and Meat
2.5. Vitamin E and Mineral Analysis
2.6. Thiobarbituric Acid Reactive Substances (TBARS)
2.7. Warner-Bratzler Shear Force (WBSF)
2.8. Statistical Analysis
3. Results
3.1. Weather Conditions
3.2. Feeds
3.2.1. Proximate Composition of Feeds
3.2.2. Fatty Acid Composition of the Feeds
3.2.3. Phytochemical Content of Feeds
3.3. Animal Performance and Carcass Traits
3.4. Beef Fatty Acids
3.4.1. Saturated and Monounsaturated Fatty Acids
3.4.2. Polyunsaturated Fatty Acids and Biohydrogenation Intermediates
3.5. Vitamin E and Minerals in Beef
3.6. Thiobarbituric Acid Reactive Substances and Warner-Bratzler Shear Force Values for Beef
4. Discussion
4.1. Feeds
4.1.1. Proximate Composition of the Feeds
4.1.2. Fatty Acid Profile of the Feeds
4.1.3. Phytochemical Content of the Feeds
4.2. Animal Performance and Carcass Traits
4.3. Beef Fatty Acids
4.3.1. Saturated Fatty Acid Content of Beef
4.3.2. Monounsaturated Fatty Acid Content of Beef
4.3.3. Polyunsaturated Fatty Acid Content of Beef
4.3.4. Biohydrogenation Intermediates in Beef
4.4. Vitamin E, TBARS, and WBSF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, R.; McMahon, P.; Norell, E.; Kissel, R.; Benz, D. Back to Grass: The Market Potential for U.S. Grassfed Beef; Stone Barns Center for Food and Agriculture: New York, NY, USA, 2017. [Google Scholar]
- Provenza, F.D.; Kronberg, S.L.; Gregorini, P. Is grassfed meat and dairy better for human and environmental health? Front. Nutr. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.; Ali, A.M.; Oladokun, S.; Wang, J.; Davis, H. Forage-fed cattle point the way forward for beef? Future Foods 2021, 3, 100012. [Google Scholar] [CrossRef]
- Stampa, E.; Schipmann-Schwarze, C.; Hamm, U. Consumer perceptions, preferences, and behavior regarding pasture-raised livestock products: A review. Food Qual. Prefer. 2020, 82, 103872. [Google Scholar] [CrossRef]
- Xue, H.; Mainville, D.; You, W.; Nayga, R.M. Consumer preferences and willingness to pay for grass-fed beef: Empirical evidence from in-store experiments. Food Qual. Prefer. 2010, 21, 857–866. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Davis, H.; Magistrali, A.; Butler, G.; Stergiadis, S. Nutritional benefits from fatty acids in organic and grass-fed beef. Foods 2022, 11, 646. [Google Scholar] [CrossRef]
- Van Elswyk, M.E.; McNeill, S.H. Impact of grass/forage feeding versus grain finishing on beef nutrients and sensory quality: The U.S. experience. Meat Sci. 2014, 96, 535–540. [Google Scholar] [CrossRef]
- Berthelot, V.; Gruffat, D. Fatty acid composition of muscles. In INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, NL, USA, 2018; p. 640. ISBN 978-690-8686-8292-8684. [Google Scholar]
- Lenighan, Y.M.; Nugent, A.P.; Moloney, A.P.; Monahan, F.J.; Walton, J.; Flynn, A.; Roche, H.M.; Mcnulty, B.A. A modelling approach to investigate the impact of consumption of three different beef compositions on human dietary fat intakes. Public Health Nutr. 2019, 23, 2373–2383. [Google Scholar] [CrossRef] [Green Version]
- Prache, S.; Martin, B.; Coppa, M. Review: Authentication of grass-fed meat and dairy products from cattle and sheep. Animal 2020, 14, 854–863. [Google Scholar] [CrossRef] [Green Version]
- Ponnampalam, E.N.; Mann, N.J.; Sinclair, A.J. Effect of feeding systems on omega-3 fatty acids, conjugated linoleic acid and trans fatty acids in Australian beef cuts: Potential impact on human health. Asia Pac. J. Clin. Nutr. 2006, 15, 21–29. [Google Scholar]
- Alfaia, C.P.M.; Alves, S.P.; Martins, S.I.V.; Costa, A.S.H.; Fontes, C.M.G.A.; Lemos, J.P.C.; Bessa, R.J.B.; Prates, J.A.M. Effect of the feeding system on intramuscular fatty acids and conjugated linoleic acid isomers of beef cattle, with emphasis on their nutritional value and discriminatory ability. Food Chem. 2009, 114, 939–946. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pighin, D.; Pazos, A.; Chamorro, V.; Paschetta, F.; Cunzolo, S.; Godoy, F.; Messina, V.; Pordomingo, A.; Grigioni, G. A contribution of beef to human health: A review of the role of the animal production systems. Sci. World J. 2016, 2016, 8681491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- van Vliet, S.; Provenza, F.D.; Kronberg, S.L. Health-promoting phytonutrients are higher in grass-fed meat and milk. Front. Sustain. Food Syst. 2021, 4, 1–20. [Google Scholar] [CrossRef]
- Krusinski, L.; Sergin, S.; Jambunathan, V.; Rowntree, J.E.; Fenton, J.I. Attention to the details: How variations in U.S. grass-fed cattle-feed supplementation and finishing date influence human health. Front. Sustain. Food Syst. 2022, 6, 851494. [Google Scholar] [CrossRef]
- Duckett, S.K.; Neel, J.P.S.; Fontenot, J.P.; Clapham, W.M. Effects of winter stocker growth rate and finishing system on: III. Tissue proximate, fatty acid, vitamin, and cholesterol content. J. Anim. Sci. 2009, 87, 2961–2970. [Google Scholar] [CrossRef] [Green Version]
- Bronkema, S.M.; Rowntree, J.E.; Jain, R.; Schweihofer, J.P.; Bitler, C.A.; Fenton, J.I. A nutritional survey of commercially available grass-finished beef. Meat Muscle Biol. 2019, 3, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.G.; Hopkins, D.L.; Schmidtke, L.; Morris, S.; Fowler, S.M. Preliminary investigation into the use of Raman spectroscopy for the verification of Australian grass and grain fed beef. Meat Sci. 2020, 160, 107970. [Google Scholar] [CrossRef]
- van Vliet, S.; Bain, J.R.; Muehlbauer, M.J.; Provenza, F.D.; Kronberg, S.L.; Pieper, C.F.; Huffman, K.M. A metabolomics comparison of plant-based meat and grass-fed meat indicates large nutritional differences despite comparable Nutrition Facts panels. Sci. Rep. 2021, 11, 13828. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, K.H.; Johnson, R.J. Alternative beef production systems: Issues and implications. Econ. Res. Serv. 2013, 1–41. Available online: https://www.ers.usda.gov/webdocs/outlooks/37473/36491_ldpm-218-01.pdf?v=7815.8 (accessed on 23 September 2022).
- American Grassfed Association. American Grassfed Association Grassfed Ruminant Standards–March 2022. 2022, pp. 1–19. Available online: https://www.americangrassfed.org/aga-grassfed-ruminant-standards/ (accessed on 15 October 2022).
- Dewhurst, R.J.; Scollan, N.D.; Youell, S.J.; Tweed, J.K.S.; Humphreys, M.O. Influence of species, cutting date and cutting interval on the fatty acid composition of grasses. Grass Forage Sci. 2001, 56, 68–74. [Google Scholar] [CrossRef]
- Khan, N.A.; Cone, J.W.; Hendriks, W.H. Stability of fatty acids in grass and maize silages after exposure to air during the feed out period. Anim. Feed Sci. Technol. 2009, 154, 183–192. [Google Scholar] [CrossRef]
- Garcia, P.T.; Pordomingo, A.; Perez, C.D.; Rios, M.D.; Sancho, A.M.; Volpi Lagreca, G.; Casal, J.J. Influence of cultivar and cutting date on the fatty acid composition of forage crops for grazing beef production in Argentina. Grass Forage Sci. 2016, 71, 235–244. [Google Scholar] [CrossRef]
- Gwin, L. Scaling-up sustainable livestock production: Innovation and challenges for grass-fed beef in the U.S. J. Sustain. Agric. 2009, 33, 189–209. [Google Scholar] [CrossRef]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. The effect of grain source and grain processing on performance of feedlot cattle: A review. J. Anim. Sci. 1997, 75, 868–879. [Google Scholar] [CrossRef] [Green Version]
- Butler, G. Manipulating dietary PUFA in animal feed: Implications for human health. Proc. Nutr. Soc. 2014, 73, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Kalač, P.; Samková, E. The effects of feeding various forages on fatty acid composition of bovine milk fat: A review. Czech J. Anim. Sci. 2010, 55, 521–537. [Google Scholar] [CrossRef] [Green Version]
- Van Ranst, G.; Fievez, V.; Vandewalle, M.; De Riek, J.; Van Bockstaele, E. Influence of herbage species, cultivar and cutting date on fatty acid composition of herbage and lipid metabolism during ensiling. Grass Forage Sci. 2009, 64, 196–207. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Glasser, F.; Doreau, M.; Maxin, G.; Baumont, R. Fat and fatty acid content and composition of forages: A meta-analysis. Anim. Feed Sci. Technol. 2013, 185, 19–34. [Google Scholar] [CrossRef]
- Baublits, R.T.; Brown, A.H., Jr.; Pohlman, F.W.; Rule, D.C.; Johnson, Z.B.; Onks, D.O.; Murrieta, C.M.; Richards, C.J.; Loveday, H.D.; Sandelin, B.A.; et al. Fatty acid and sensory characteristics of beef from three biological types of cattle grazing cool-season forages supplemented with soyhulls. Meat Sci. 2006, 72, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Krusinski, L.; Maciel, I.C.F.; Sergin, S.; Goeden, T.; Ali, H.; Kesamneni, S.; Jambunathan, V.; Cassida, K.A.; Singh, S.; Medina-Meza, I.G.; et al. Evaluation of fatty acid and antioxidant variation in a complex pasture system as compared to standard cattle feed in the Great Lakes Region. Front. Sustain. Food Syst. 2022, 6, 945080. [Google Scholar] [CrossRef]
- Maciel, I.C.F.; Schweihofer, J.P.; Fenton, J.I.; Hodbod, J.; McKendree, M.G.S.; Cassida, K.; Rowntree, J.E. Influence of beef genotypes on animal performance, carcass traits, meat quality, and sensory characteristics in grazing or feedlot-finished steers. Transl. Anim. Sci. 2021, 5, txab214. [Google Scholar] [CrossRef]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Hach, C.C.; Bowden, B.K.; Kopelove, A.B.; Brayton, S.V. More powerful peroxide Kjeldahl digestion method. J. Assoc. Off. Anal. Chem. 1987, 70, 783–787. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 2011, 129, 155–161. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Sergin, S.; Goeden, T.; Krusinski, L.; Kesamneni, S.; Ali, H.; Bitler, C.A.; Medina-Meza, I.G.; Fenton, J.I. Fatty acid and antioxidant composition of conventional compared to pastured eggs: Characterization of conjugated linoleic acid and branched chain fatty acid isomers in eggs. Food Sci. Technol. 2021, 1, 260–267. [Google Scholar] [CrossRef]
- Jenkins, T.C. Technical note: Common analytical errors yielding inaccurate results during analysis of fatty acids in feed and digesta samples. J. Dairy Sci. 2010, 93, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.K.; Hernandez, M.; Cruz-Hernandez, C.; Kraft, J.; Dugan, M.E. Combining results of two GC separations partly achieves determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-ion SPE fractionation. Lipids 2008, 43, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Rettenmaier, R.; Schüep, W. Determination of vitamins A and E in liver tissue. Int. J. Vitam. Nutr. Res. 1992, 62, 312–317. [Google Scholar]
- Wahlen, R.; Evans, L.; Turner, J.; Hearn, R. The use of collision/reaction cell ICP-MS for the determination of elements in blood and serum samples. Spectroscopy 2005, 20, 84–89. [Google Scholar]
- Krusinski, L.; Maciel, I.C.F.; Sergin, S.; Goeden, T.; Schweihofer, J.P.; Singh, S.; Rowntree, J.E.; Fenton, J.I. Fatty acid and micronutrient profile of longissimus lumborum from Red Angus and Red Angus x Akaushi cattle finished on grass or grain. Foods 2022, 11, 3451. [Google Scholar] [CrossRef]
- Commission Regulation of European Union. Amending Regulation (EC) No 1924/2006 of the European Parliament and of the Council with regard to the list of nutrition claims. Off. J. Eur. Union 2010, 116. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:037:0016:0018:EN:PDF (accessed on 29 September 2022).
- Booth, J.; Howard, T.; Miller-Auwerda, P.; Moore, J. Soybean Hulls as an Alternative Feed for Horses; Animal Industry Report; Iowa State University: Ames, IA, USA, 2004; p. ASL R1931. [Google Scholar]
- Poore, M.H.; Johns, J.T.; Burris, W.R. Soybean hulls, wheat middlings, and corn gluten feed as supplements for cattle on forage-based diets. Vet. Clin. N. Am. Food Anim. Pract. 2002, 18, 213–231. [Google Scholar] [CrossRef]
- Scollan, N.D.; Dannenberger, D.; Nuernberg, K.; Richardson, I.; MacKintosh, S.; Hocquette, J.F.; Moloney, A.P. Enhancing the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2014, 97, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Elgersma, A.; Søegaard, K.; Jensen, S.K. Fatty acids, α-tocopherol, β-carotene, and lutein contents in forage legumes, forbs, and a grass-clover mixture. J. Agric. Food Chem. 2013, 61, 11913–11920. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, T.F.; Mannion, D.; Apopei, D.; McCarthy, N.A.; Hogan, S.A.; Kilcawley, K.N.; Egan, M. Influence of supplemental feed choice for pasture-based cows on the fatty acid and volatile profile of milk. Foods 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalač, P. The effects of feeding fresh forage and silage on some nutritional attributes of beef: An overview. J. Agrobiol. 2011, 28, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Cone, J.W.; Fievez, V.; Hendriks, W.H. Causes of variation in fatty acid content and composition in grass and maize silages. Anim. Feed Sci. Technol. 2012, 174, 36–45. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Khadka, D.; Timilsina, R. Total phenolic contents and antioxidant activity profile of selected cereal sprouts and grasses. Int. J. Food Prop. 2019, 22, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, H.P.; Singh, A.P.; Upadhyay, V.S.; Kessels, H.P.P.; Harika, A.S.; Singh, S.; Ibrahim, M.N.M. Forage conservation, storage and feeding. In Handbook for Straw Feeding System; Singh, K., Schiere, J.B., Eds.; ICAR: New Delhi, India, 1995; pp. 303–323. [Google Scholar]
- Liu, S.X.; Chen, D.; Singh, M.; Xu, J. Extraction of proteins and pasting and antioxidant properties of soybean hulls. J. Food Res. 2019, 8, 66–77. [Google Scholar] [CrossRef]
- Peiretti, P.; Karamać, M.; Janiak, M.; Longato, E.; Meineri, G.; Amarowicz, R.; Gai, F. Phenolic composition and antioxidant activities of soybean (Glycine max (L.) Merr.) plant during growth cycle. Agronomy 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Neel, J.P.; Fontenot, J.P.; Clapham, W.M.; Duckett, S.K.; Felton, E.E.; Scaglia, G.; Bryan, W.B. Effects of winter stocker growth rate and finishing system on: I. Animal performance and carcass characteristics. J. Anim. Sci. 2007, 85, 2012–2018. [Google Scholar] [CrossRef] [Green Version]
- Dennis, T.S.; Tower, J.E.; Nennich, T.D. Effects of feeding hay and baleage to prepubertal dairy heifers during the grower period. Prof. Anim. Sci. 2012, 28, 648–656. [Google Scholar] [CrossRef]
- Casperson, S.L.; Conrad, Z.; Raatz, S.K.; Derner, J.; Roemmich, J.N.; Jahns, L.; Picklo, M.J. Impact of beef consumption on saturated fat intake in the United States adult population: Insights from modeling the influences of bovine genetics and nutrition. Meat Sci. 2020, 169, 108225. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Carbone, S.; Lavie, C.J. Dietary fats and chronic noncommunicable diseases. Nutrients 2018, 10, 1385. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2020.
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enteral Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- FAO. Fats and fatty acids in human nutrition: Report of an expert consultation. FAO Food Nutr. Pap. 2010, 91, 1–166. [Google Scholar]
- Loften, J.R.; Linn, J.G.; Drackley, J.K.; Jenkins, T.C.; Soderholm, C.G.; Kertz, A.F. Invited review: Palmitic and stearic acid metabolism in lactating dairy cows. J. Dairy Sci. 2014, 97, 4661–4674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leheska, J.M.; Thompson, L.D.; Howe, J.C.; Hentges, E.; Boyce, J.; Brooks, J.C.; Shriver, B.; Hoover, L.; Miller, M.F. Effects of conventional and grass-feeding systems on the nutrient composition of beef. J. Anim. Sci. 2008, 86, 3575–3585. [Google Scholar] [CrossRef] [Green Version]
- Duckett, S.K.; Neel, J.P.S.; Lewis, R.M.; Fontenot, J.P.; Clapham, W.M. Effects of forage species or concentrate finishing on animal performance, carcass and meat quality. J. Anim. Sci. 2013, 91, 1454–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-bello-pérez, E.; Garnsworthy, P.C. Trans fatty acids and their role in the milk of dairy cows. Cien. Inv. Agr. 2013, 40, 449–473. [Google Scholar] [CrossRef]
- French, P.; Stanton, C.; Lawless, F.; O’Riordan, E.G.; Monahan, F.J.; Caffrey, P.J.; Moloney, A.P. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets. J. Anim. Sci. 2000, 78, 2849–2855. [Google Scholar] [CrossRef]
- Kraft, J.; Kramer, J.K.G.; Schoene, F.; Chambers, J.R.; Jahreis, G. Extensive analysis of long-chain polyunsaturated fatty acids, CLA, trans-18-1 isomers, and plasmalogenic lipids in different retail beef types. J. Agric. Food Chem. 2008, 56, 4775–4782. [Google Scholar] [CrossRef]
- Gebauer, S.K.; Destaillats, F.; Dionisi, F.; Krauss, R.M.; Baer, D.J. Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect LDL cholesterol: A double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Verneque, B.J.F.; Machado, A.M.; de Abreu Silva, L.; Lopes, A.C.S.; Duarte, C.K. Ruminant and industrial trans-fatty acids consumption and cardiometabolic risk markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 62, 2050–2060. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Shingfield, K.J.; Lee, M.R.F.; Scollan, N.D. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed Sci. Technol. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Howe, P.; Meyer, B.; Record, S.; Baghurst, K. Dietary intake of long-chain ω-3 polyunsaturated fatty acids: Contribution of meat sources. Nutrition 2006, 22, 47–53. [Google Scholar] [CrossRef]
- Parolini, C. Effects of fish n-3 PUFAs on intestinal microbiota and immune system. Mar. Drugs 2019, 17, 374. [Google Scholar] [CrossRef] [Green Version]
- Mendivil, C.O. Dietary fish, fish nutrients, and immune function: A review. Front. Nutr. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Byelashov, O.A.; Sinclair, A.J.; Kaur, G. Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technol. 2015, 27, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Hunde, Y.; Patton, J.; Padgitt, D.; Vlieger, D. Particle size influences preferential consumption of hay and baleage in total mixed dairy rations. Trans. Mo. Acad. Sci. 2008, 42, 45–49. [Google Scholar] [CrossRef]
- Scollan, N.D.; Hocquette, J.F.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef]
- Lock, A.L.; Harvatine, K.J.; Drackley, J.K.; Bauman, D.E. Concepts in fat and fatty acid digestion in ruminants. In Proceedings of the Intermountain Nutrition Conference, Utah State University, Logan, UT, USA, January 2006; pp. 85–100. [Google Scholar]
- Kiesling, D.D. Performance, Carcass Traits and Fatty Acid Profiles of Yearling Beef Cattle Supplemented with Self-Fed Byproducts on Pasture; Iowa State University: Ames, IA, USA, 2013. [Google Scholar]
- De la Fuente, J.; Diaz, M.T.; Alvarez, I.; Oliver, M.A.; Font, I.F.M.; Sanudo, C.; Campo, M.M.; Montossi, F.; Nute, G.R.; Caneque, V. Fatty acid and vitamin E composition of intramuscular fat in cattle reared in different production systems. Meat Sci. 2009, 82, 331–337. [Google Scholar] [CrossRef]
- Greene, B.E.; Cumuze, T.H. Relationship between TBA numbers and inexperienced panelists’ assessments of oxidized flavor in cooked beef. J. Food Sci. 1981, 47, 52–54. [Google Scholar] [CrossRef]
- Nuernberg, K.; Dannenberger, D.; Nuernberg, G.; Ender, K.; Voigt, J.; Scollan, N.D.; Wood, J.D.; Nute, G.R.; Richardson, R.I. Effect of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid composition of longissimus muscle in different cattle breeds. Livest. Prod. Sci. 2005, 94, 137–147. [Google Scholar] [CrossRef]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “grass-fed" milk story: Understanding the impact of pasture feeding on the composition and quality of bovine milk. Foods 2019, 8, 350. [Google Scholar] [CrossRef]




| GRASS | HAY | BLG 1 | SH 2 | p-Value | |
|---|---|---|---|---|---|
| DM 3 | 57.52 ± 22.47 | 85.82 ± 25.01 | 82.64 ± 25.02 | 89.98 ± 25.01 | 0.159 |
| Ash * | 9.20 ± 0.46 a | 7.14 ± 0.61 b | 8.38 ± 0.63 a,b | 4.74 ± 0.61 c | <0.001 |
| CP 4 * | 15.65 ± 0.39 a | 7.15 ± 1.14 c | 13.48 ± 1.19 a,b | 9.47 ± 1.19 b,c | <0.001 |
| NDF 5 * | 54.91 ± 3.30 b | 66.19 ± 4.01 a,b | 54.23 ± 4.04 b | 68.29 ± 4.01 a | 0.004 |
| ADF 6 * | 30.84 ± 0.72 c | 37.98 ± 1.35 b | 33.51 ± 1.39 b,c | 51.72 ± 1.35 a | <0.001 |
| Energy 7 | 4566.41 ± 50.99 a | 4405.00 ± 90.76 a | 4465.41 ± 50.99 a | 3709.96 ± 90.76 b | <0.001 |
| GRASS | HAY | BLG 1 | SH 2 | p-Value | |
|---|---|---|---|---|---|
| C10:0 | 0.16 ± 0.26 | 1.16 ± 0.38 | 0.14 ± 0.38 | 0.03 ± 0.38 | 0.116 |
| C12:0 | 0.50 ± 0.22 b | 1.16 ± 0.23 a | 0.49 ± 0.23 b | 0.10 ± 0.23 c | <0.001 |
| C13:0 | 0.01 ± 0.02 | 0.07 ± 0.03 | 0.02 ± 0.03 | 0.00 ± 0.03 | 0.193 |
| C14:0 | 0.53 ± 0.29 b | 2.14 ± 0.45 a | 0.62 ± 0.45 a,b | 0.21 ± 0.45 b | 0.041 |
| C15:0 | 0.11 ± 0.06 b | 0.49 ± 0.09 a | 0.33 ± 0.09 a,b | 0.14 ± 0.09 b | 0.010 |
| C16:0 | 14.29 ± 1.98 c | 32.16 ± 2.37 a | 23.77 ± 2.38 b | 14.95 ± 2.37 c | <0.001 |
| C16:1 9c | 0.23 ± 0.02 b | 0.51 ± 0.04 a | 0.31 ± 0.04 b | 0.23 ± 0.04 b | 0.001 |
| C16:1 7c | 1.19 ± 0.27 a | 1.21 ± 0.29 a | 1.52 ± 0.29 a | 0.11 ± 0.29 b | <0.001 |
| C17:0 | 0.21 ± 0.02 c | 0.57 ± 0.03 a | 0.36 ± 0.03 b | 0.28 ± 0.03 b,c | <0.001 |
| C18:0 | 1.57 ± 0.13 c | 3.80 ± 0.20 a | 2.78 ± 0.20 b | 4.33 ± 0.20 a | <0.001 |
| C18:1 9c | 1.84 ± 0.07 c | 3.45 ± 0.23 b | 2.43 ± 0.24 b,c | 12.61 ± 0.23 a | <0.001 |
| C18:1 11c | 0.61 ± 0.03 c | 1.04 ± 0.09 b | 0.72 ± 0.10 b,c | 2.43 ± 0.09 a | <0.001 |
| C18:2 n-6 (LA) 3 | 12.22 ± 0.35 c | 14.86 ± 0.88 b,c | 16.42 ± 0.91 b | 48.45 ± 0.88 a | <0.001 |
| C18:3 n-3 (ALA) 4 | 64.66 ± 1.74 a | 32.48 ± 2.57 c | 46.47 ± 2.64 b | 15.00 ± 2.57 d | <0.001 |
| C20:0 | 0.58 ± 0.08 b | 2.72 ± 0.16 a | 1.05 ± 0.16 b | 0.41 ± 0.16 b | <0.001 |
| C20:3 n-3 | 0.08 ± 0.03 | 0.03 ± 0.03 | 0.05 ± 0.03 | 0.01 ± 0.03 | 0.052 |
| C22:0 | 0.59 ± 0.16 b,c | 1.90 ± 0.20 a | 1.04 ± 0.21 b | 0.29 ± 0.20 c | <0.001 |
| C24:0 | 0.61 ± 0.32 b | 1.78 ± 0.36 a | 1.40 ± 0.36 a | 0.24 ± 0.36 b | 0.001 |
| ∑ SFA 5 | 19.17 ± 2.41 c | 48.03 ± 3.28 a | 31.86 ± 3.29 b | 21.01 ± 3.28 b,c | <0.001 |
| ∑ OCFA 6 | 0.33 ± 0.06 c | 1.11 ± 0.10 a | 0.71 ± 0.10 a,b | 0.44 ± 0.10 b,c | <0.001 |
| ∑ MUFA 7 | 3.87 ± 0.30 c | 6.18 ± 0.39 b | 4.96 ± 0.40 b | 15.40 ± 0.39 a | <0.001 |
| ∑ PUFA 8 | 76.96 ± 2.72 a | 45.79 ± 3.56 c | 63.11 ± 3.58 b | 63.51 ± 3.56 b | <0.001 |
| ∑ n-6 9 | 12.22 ± 0.35 c | 14.86 ± 0.88 b,c | 16.42 ± 0.91 b | 48.45 ± 0.88 a | <0.001 |
| ∑ n-3 10 | 64.73 ± 1.71 a | 32.51 ± 2.55 c | 46.52 ± 2.62 b | 15.02 ± 2.55 d | <0.001 |
| n-6:n-3 ratio 11 | 0.19 ± 0.04 c | 0.53 ± 0.07 b | 0.38 ± 0.07 b,c | 3.20 ± 0.07 a | <0.001 |
| G-HAY 1 | G-BLG 2 | G-SH 3 | BLG-SH 4 | p-Value | |
|---|---|---|---|---|---|
| Growth (kg) | |||||
| Initial BW 5 | 388.70 ± 30.30 | 390.77 ± 30.33 | 388.77 ± 30.36 | 378.27 ± 30.30 | 0.560 |
| Final BW | 483.27 ± 8.63 c | 493.74 ± 8.77 b,c | 524.33 ± 8.92 a,b | 536.31 ± 8.63 a | <0.001 |
| Total gain | 94.57 ± 25.64 b | 103.02 ± 25.65 b | 135.77 ± 25.66 a | 158.04 ± 25.64 a | <0.001 |
| ADG 6 | 0.61 ± 0.10 b | 0.66 ± 0.10 b | 0.88 ± 0.10 a | 1.03 ± 0.10 a | <0.001 |
| Carcass | |||||
| HCW 7 (kg) | 281.85 ± 5.53 c | 287.05 ± 5.62 b,c | 311.11 ± 5.72 a | 306.53 ± 5.53 a,b | 0.003 |
| Backfat (mm) | 7.15 ± 0.75 a,b | 5.91 ± 0.76 b | 9.18 ± 0.77 a | 9.38 ± 0.75 a | 0.011 |
| Ribeye area (cm2) | 68.28 ± 2.19 b | 75.99 ± 2.20 a | 75.57 ± 2.22 a | 76.41 ± 2.19 a | 0.012 |
| USDA yield grade | 2.80 ± 0.30 a,b | 2.10 ± 0.31 b | 3.29 ± 0.31 a | 2.80 ± 0.30 a,b | 0.032 |
| Marbling score 8 | 348.00 ± 11.66 a,b | 332.55 ± 11.81 b | 387.39 ± 11.97 a | 392.00 ± 11.66 a | 0.004 |
| G-HAY 1 | G-BLG 2 | G-SH 3 | BLG-SH 4 | p-Value | |
|---|---|---|---|---|---|
| ∑ SFA 5 | 275.08 ± 40.89 | 272.87 ± 41.46 | 332.71 ± 42.05 | 356.22 ± 40.89 | 0.400 |
| C10:0 | 1.75 ± 1.92 | 2.54 ± 1.92 | 3.17 ± 1.92 | 3.28 ± 1.92 | 0.163 |
| C12:0 | 0.57 ± 0.31 | 0.60 ± 0.31 | 0.73 ± 0.31 | 0.70 ± 0.31 | 0.442 |
| C13:0 | 0.09 ± 0.07 | 0.11 ± 0.07 | 0.12 ± 0.07 | 0.11 ± 0.07 | 0.247 |
| C14:0 | 12.40 ± 2.29 | 12.04 ± 2.33 | 14.95 ± 2.36 | 15.98 ± 2.29 | 0.566 |
| C15:0 | 2.00 ± 0.31 | 2.11 ± 0.31 | 1.96 ± 0.32 | 2.02 ± 0.31 | 0.988 |
| C16:0 | 162.66 ± 24.15 | 162.29 ± 24.49 | 202.57 ± 24.83 | 220.59 ± 24.15 | 0.259 |
| C17:0 | 4.65 ± 0.79 | 4.86 ± 0.81 | 5.52 ± 0.82 | 6.01 ± 0.79 | 0.617 |
| C18:0 | 87.03 ± 14.33 | 84.13 ± 14.49 | 99.28 ± 14.66 | 103.06 ± 14.33 | 0.694 |
| C19:0 | 1.91 ± 1.54 | 2.81 ± 1.55 | 2.84 ± 1.55 | 2.99 ± 1.54 | 0.270 |
| C20:0 | 0.78 ± 0.35 | 0.67 ± 0.35 | 0.81 ± 0.35 | 0.81 ± 0.35 | 0.305 |
| C22:0 | 1.23 ± 0.39 a | 0.73 ± 0.39 b | 0.79 ± 0.39 b | 0.84 ± 0.39 b | <0.001 |
| ∑ BCFA 6 | 11.11 ± 1.63 | 11.41 ± 1.65 | 13.08 ± 1.68 | 12.29 ± 1.63 | 0.831 |
| C14:0 iso | 0.15 ± 0.06 | 0.19 ± 0.06 | 0.17 ± 0.06 | 0.16 ± 0.06 | 0.863 |
| C15:0 iso | 0.73 ± 0.16 | 0.77 ± 0.16 | 0.94 ± 0.16 | 0.84 ± 0.16 | 0.771 |
| C15:0 anteiso | 0.69 ± 0.15 | 0.72 ± 0.15 | 0.73 ± 0.15 | 0.67 ± 0.15 | 0.979 |
| C16:0 iso | 0.77 ± 0.25 | 0.77 ± 0.25 | 0.82 ± 0.25 | 0.80 ± 0.25 | 0.989 |
| C17:0 iso | 4.11 ± 0.45 | 4.16 ± 0.46 | 4.81 ± 0.47 | 4.21 ± 0.45 | 0.689 |
| C17:0 anteiso | 4.06 ± 0.68 | 4.27 ± 0.69 | 4.96 ± 0.70 | 4.96 ± 0.68 | 0.717 |
| C18:0 iso | 0.60 ± 0.19 | 0.54 ± 0.20 | 0.65 ± 0.20 | 0.65 ± 0.19 | 0.819 |
| ∑ MUFA 7 | 313.55 ± 39.66 | 313.17 ± 40.22 | 381.51 ± 40.81 | 371.90 ± 39.66 | 0.489 |
| ∑ cMUFA 8 | 276.91 ± 36.21 | 272.67 ± 36.72 | 342.84 ± 37.26 | 342.10 ± 36.21 | 0.358 |
| C14:1 9c | 2.67 ± 0.52 | 2.78 ± 0.53 | 3.36 ± 0.53 | 3.13 ± 0.52 | 0.724 |
| C16:1 9c | 36.73 ± 4.92 | 35.81 ± 5.00 | 45.02 ± 5.09 | 44.15 ± 4.92 | 0.444 |
| C16:1 10c | 4.17 ± 1.55 | 5.19 ± 1.55 | 4.51 ± 1.55 | 4.16 ± 1.55 | 0.554 |
| C16:1 11c | 2.00 ± 1.39 | 2.52 ± 1.39 | 2.66 ± 1.39 | 2.76 ± 1.39 | 0.328 |
| C17:1 9c | 3.76 ± 0.43 | 3.98 ± 0.43 | 4.35 ± 0.44 | 4.50 ± 0.43 | 0.490 |
| C18:1 9c | 199.97 ± 34.69 | 196.83 ± 35.01 | 252.79 ± 35.35 | 255.23 ± 34.69 | 0.309 |
| C18:1 11c | 11.87 ± 1.50 | 10.09 ± 1.51 | 12.75 ± 1.52 | 11.57 ± 1.50 | 0.321 |
| C18:1 12c | 2.14 ± 0.61 | 2.12 ± 0.61 | 2.44 ± 0.61 | 2.46 ± 0.61 | 0.560 |
| C18:1 13c | 2.33 ± 1.60 | 3.11 ± 1.61 | 3.64 ± 1.61 | 3.72 ± 1.60 | 0.065 |
| C18:1 14c | 0.91 ± 0.26 | 0.83 ± 0.26 | 0.90 ± 0.26 | 0.90 ± 0.26 | 0.878 |
| C18:1 15c | 1.29 ± 0.83 | 1.74 ± 0.83 | 1.81 ± 0.83 | 1.95 ± 0.83 | 0.153 |
| C20:1 9c | 2.82 ± 1.70 | 2.67 ± 1.71 | 3.08 ± 1.71 | 2.92 ± 1.70 | 0.718 |
| C20:1 11c | 6.26 ± 1.44 | 4.88 ± 1.44 | 5.51 ± 1.44 | 4.63 ± 1.44 | 0.179 |
| ∑ tMUFA 9 | 36.64 ± 9.96 | 40.71 ± 9.99 | 38.88 ± 10.02 | 29.80 ± 9.96 | 0.479 |
| C16:1 9t | 6.06 ± 1.66 a | 6.68 ± 1.66 a | 6.11 ± 1.67 a | 3.82 ± 1.66 b | 0.003 |
| C16:1 10,11,12t | 5.49 ± 2.51 | 6.99 ± 2.52 | 6.83 ± 2.52 | 6.63 ± 2.51 | 0.373 |
| C18:1 6-8t | 1.56 ± 0.99 | 2.07 ± 0.99 | 1.83 ± 0.99 | 2.08 ± 0.99 | 0.181 |
| C18:1 9t | 1.62 ± 1.19 | 2.44 ± 1.19 | 2.51 ± 1.19 | 2.52 ± 1.19 | 0.221 |
| C18:1 10t | 1.35 ± 1.23 | 2.49 ± 1.23 | 2.17 ± 1.23 | 1.83 ± 1.23 | 0.149 |
| C18:1 11t | 13.77 ± 2.73 | 12.37 ± 2.77 | 11.73 ± 2.80 | 5.64 ± 2.73 | 0.196 |
| C18:1 12t | 1.36 ± 0.51 | 1.43 ± 0.51 | 1.53 ± 0.51 | 1.26 ± 0.51 | 0.755 |
| C18:1 13,14t | 2.92 ± 0.31 | 2.72 ± 0.31 | 2.73 ± 0.32 | 2.51 ± 0.31 | 0.832 |
| C18:1 15t | 1.44 ± 1.65 | 2.24 ± 1.65 | 2.60 ± 1.65 | 2.34 ± 1.65 | 0.079 |
| C18:1 16t | 1.36 ± 0.35 | 1.29 ± 0.35 | 1.27 ± 0.35 | 1.17 ± 0.35 | 0.918 |
| ∑ FA 10 | 729.92 ± 83.69 | 698.46 ± 84.92 | 833.36 ± 86.21 | 840.46 ± 83.69 | 0.550 |
| G-HAY 1 | G-BLG 2 | G-SH 3 | BLG-SH 4 | p-Value | |
|---|---|---|---|---|---|
| ∑ PUFA 5 | 98.31 ± 3.50 a | 72.54 ± 3.56 b | 76.57 ± 3.63 b | 73.69 ± 3.50 b | <0.001 |
| ∑ n-3 6 | 47.29 ± 2.97 a | 29.04 ± 2.99 b | 26.57 ± 3.01 b | 27.20 ± 2.97 b | <0.001 |
| C18:3 n-3 (ALA) 7 | 10.63 ± 1.26 a | 10.39 ± 1.26 a,b | 8.62 ± 1.27 a,b | 8.31 ± 1.26 b | 0.014 |
| C20:3 n-3 | 0.89 ± 0.41 | 0.68 ± 0.41 | 0.74 ± 0.41 | 0.70 ± 0.41 | 0.235 |
| C20:5 n-3 (EPA) 8 | 9.26 ± 0.38 a | 5.68 ± 0.39 b | 5.04 ± 0.39 b | 5.00 ± 0.38 b | <0.001 |
| C22:5 n-3 (DPA) 9 | 24.18 ± 2.82 a | 11.09 ± 2.82 b | 10.88 ± 2.83 b | 11.59 ± 2.82 b | <0.001 |
| C22:6 n-3 (DHA) 10 | 2.33 ± 0.54 a | 1.43 ± 0.54 b | 1.51 ± 0.54 b | 1.60 ± 0.54 b | <0.001 |
| ∑ n-6 11 | 47.64 ± 2.84 | 41.67 ± 2.86 | 48.26 ± 2.88 | 44.67 ± 2.84 | 0.108 |
| C18:2 n-6 (LA) 12 | 28.23 ± 3.73 | 25.42 ± 3.73 | 30.14 ± 3.74 | 27.37 ± 3.73 | 0.121 |
| C18:3 n-6 | 0.72 ± 0.46 | 0.69 ± 0.46 | 0.76 ± 0.46 | 0.76 ± 0.46 | 0.733 |
| C20:2 n-6 | 1.01 ± 0.32 | 0.76 ± 0.32 | 0.84 ± 0.32 | 0.90 ± 0.32 | 0.087 |
| C20:3 n-6 | 2.20 ± 0.31 | 2.21 ± 0.31 | 2.65 ± 0.31 | 2.53 ± 0.31 | 0.123 |
| C20:4 n-6 | 10.43 ± 1.19 | 10.02 ± 1.20 | 10.63 ± 1.20 | 10.02 ± 1.19 | 0.903 |
| C22:4 n-6 | 5.05 ± 1.36 a | 2.68 ± 1.36 b | 3.35 ± 1.36 b | 3.29 ± 1.36 b | <0.001 |
| n-6:n-3 ratio 13 | 1.03 ± 0.23 c | 1.49 ± 0.23 b | 1.89 ± 0.23 a | 1.70 ± 0.23 a,b | <0.001 |
| C20:3 n-9 | 3.38 ± 0.93 a | 1.87 ± 0.93 b | 1.79 ± 0.93 b | 1.83 ± 0.93 b | <0.001 |
| ∑ CLnA 14 | 1.59 ± 1.15 | 1.56 ± 1.15 | 1.76 ± 1.15 | 1.63 ± 1.15 | 0.690 |
| C18:3 9c,11t,15t | 0.80 ± 0.57 | 0.82 ± 0.57 | 0.91 ± 0.57 | 0.84 ± 0.57 | 0.676 |
| C18:3 9c,11t,15c | 0.79 ± 0.58 | 0.74 ± 0.58 | 0.85 ± 0.58 | 0.79 ± 0.58 | 0.648 |
| ∑ AD 15 | 18.30 ± 6.84 | 17.25 ± 6.84 | 17.56 ± 6.85 | 15.84 ± 6.84 | 0.833 |
| C18:2 11t,15t | 4.26 ± 0.84 | 3.27 ± 0.84 | 3.22 ± 0.85 | 2.65 ± 0.84 | 0.207 |
| C18:2 9t,12t | 1.82 ± 1.09 | 2.27 ± 1.09 | 2.37 ± 1.09 | 2.21 ± 1.09 | 0.377 |
| C18:2 9c,14t/9c,13t | 2.60 ± 1.27 | 2.69 ± 1.27 | 2.78 ± 1.27 | 2.56 ± 1.27 | 0.944 |
| C18:2 11t,15c | 4.20 ± 0.82 | 3.48 ± 0.82 | 3.04 ± 0.83 | 2.52 ± 0.82 | 0.189 |
| C18:2 9c,16t | 1.90 ± 0.88 | 1.87 ± 0.88 | 2.07 ± 0.88 | 2.00 ± 0.88 | 0.715 |
| C18:2 9c,15c | 2.10 ± 1.29 | 2.35 ± 1.29 | 2.59 ± 1.29 | 2.39 ± 1.29 | 0.587 |
| C18:2 12c,15c | 1.42 ± 0.80 | 1.31 ± 0.80 | 1.48 ± 0.80 | 1.51 ± 0.80 | 0.589 |
| ∑ CLA 16 | 10.45 ± 3.43 | 8.35 ± 3.44 | 9.03 ± 3.44 | 7.11 ± 3.43 | 0.107 |
| C18:2 9c,11t/9c,7t | 6.26 ± 1.00 a | 4.41 ± 1.00 a,b | 4.66 ± 1.00 a,b | 3.05 ± 1.00 b | 0.015 |
| C18:2 11t,13c | 1.72 ± 0.89 | 1.51 ± 0.89 | 1.64 ± 0.89 | 1.49 ± 0.89 | 0.570 |
| C18:2 11t,13t | 1.27 ± 0.83 | 1.28 ± 0.83 | 1.44 ± 0.83 | 1.36 ± 0.83 | 0.580 |
| C18:2 t,t | 1.20 ± 0.80 | 1.15 ± 0.80 | 1.27 ± 0.80 | 1.21 ± 0.80 | 0.799 |
| G-HAY 1 | G-BLG 2 | G-SH 3 | BLG-SH 4 | p-Value | |
|---|---|---|---|---|---|
| Vitamin E | 29.93 ± 1.44 a | 28.86 ± 1.46 a | 25.62 ± 1.47 a | 13.83 ± 1.44 b | <0.001 |
| Selenium | 0.44 ± 0.04 | 0.42 ± 0.04 | 0.44 ± 0.04 | 0.45 ± 0.04 | 0.561 |
| Iron | 59.87 ± 7.61 | 59.65 ± 7.61 | 60.41 ± 7.62 | 56.94 ± 7.61 | 0.422 |
| Copper | 1.98 ± 0.07 | 2.09 ± 0.07 | 2.07 ± 0.07 | 1.93 ± 0.07 | 0.117 |
| Zinc | 126.31 ± 3.35 | 123.35 ± 3.40 | 123.80 ± 3.45 | 119.36 ± 3.35 | 0.545 |
| Manganese | 0.92 ± 0.02 a | 0.90 ± 0.02 a,b | 0.85 ± 0.02 b,c | 0.84 ± 0.02 c | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krusinski, L.; Maciel, I.C.F.; Sergin, S.; Jambunathan, V.; Garg, E.; Garmyn, A.J.; Singh, S.; Bitler, C.A.; Rowntree, J.E.; Fenton, J.I. Effects of Hay, Baleage, and Soybean Hulls Waste Used as Supplemental Feeds on the Nutritional Profile of Grass-Finished Beef. Foods 2022, 11, 3856. https://doi.org/10.3390/foods11233856
Krusinski L, Maciel ICF, Sergin S, Jambunathan V, Garg E, Garmyn AJ, Singh S, Bitler CA, Rowntree JE, Fenton JI. Effects of Hay, Baleage, and Soybean Hulls Waste Used as Supplemental Feeds on the Nutritional Profile of Grass-Finished Beef. Foods. 2022; 11(23):3856. https://doi.org/10.3390/foods11233856
Chicago/Turabian StyleKrusinski, Lucas, Isabella C. F. Maciel, Selin Sergin, Vijayashree Jambunathan, Esha Garg, Andrea J. Garmyn, Sukhdeep Singh, Chad A. Bitler, Jason E. Rowntree, and Jenifer I. Fenton. 2022. "Effects of Hay, Baleage, and Soybean Hulls Waste Used as Supplemental Feeds on the Nutritional Profile of Grass-Finished Beef" Foods 11, no. 23: 3856. https://doi.org/10.3390/foods11233856
APA StyleKrusinski, L., Maciel, I. C. F., Sergin, S., Jambunathan, V., Garg, E., Garmyn, A. J., Singh, S., Bitler, C. A., Rowntree, J. E., & Fenton, J. I. (2022). Effects of Hay, Baleage, and Soybean Hulls Waste Used as Supplemental Feeds on the Nutritional Profile of Grass-Finished Beef. Foods, 11(23), 3856. https://doi.org/10.3390/foods11233856