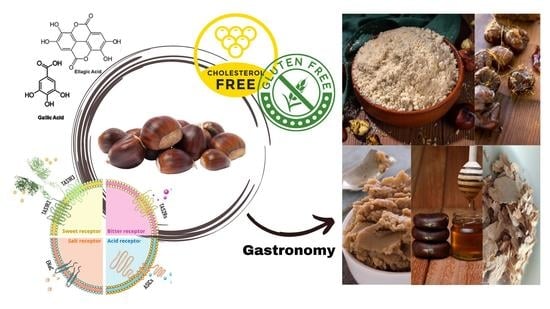
Sweet Chestnut (Castanea sativa Mill.) Nutritional and Phenolic Composition Interactions with Chestnut Flavor Physiology
Abstract
:1. Introduction
2. Chestnut Chemical Composition
3. Chestnut Nutritional and Health Benefits
4. Chestnut Sensory Pleasantness
4.1. Chestnut Taste and Flavor
4.2. Trigeminal Sensations and Chestnut Palatability
4.3. Olfactory Sensations and Chestnut Aroma
5. Methods and Sensory Lexicons Used on Chestnut Sensory and Qualitative Evaluation
6. Chestnut in Gastronomy
7. Final Remarks
8. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lang, P.; Dane, F.; Kubisiak, T.L.; Huang, H. Molecular evidence for an Asian origin and a unique westward migration of species in the genus Castanea via Europe to North America. Mol. Phylogenet. Evol. 2007, 43, 49–59. [Google Scholar] [CrossRef]
- Wani, I.A.; Hamid, H.; Hamdani, A.M.; Gani, A.; Ashwar, B.A. Physico-chemical, rheological and antioxidant properties of sweet chestnut (Castanea sativa Mill.) as affected by pan and microwave roasting. J. Adv. Res. 2017, 8, 399–405. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geana, E.I.; Sandru, C.; Tita, O.; Botu, M. Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania. Separations 2022, 9, 66. [Google Scholar] [CrossRef]
- Künsch, U.; Schärer, H.; Patrian, B.; Höhn, E.; Conedera, M.; Sassella, A.; Jermini, M.; Jelmini, G. Effects of roasting on chemical composition and quality of different chestnut (Castanea sativa Mill) varieties. J. Sci. Food Agric. 2001, 81, 1106–1112. [Google Scholar] [CrossRef]
- Correia, P.; Leitão, A.; Beirão-da-Costa, M.L. The effect of drying temperatures on morphological and chemical properties of dried chestnuts flours. J. Food Eng. 2009, 90, 325–332. [Google Scholar] [CrossRef]
- Silva, A.P.; Santos-Ribeiro, R.; Borges, O.; Magalhães, B.; Silva, M.E.; Gonçalves, B. Effects of roasting and boiling on the physical and mechanical properties of 11 Portuguese chestnut cultivars (Castanea sativa Mill.). CYTA—J. Food 2011, 9, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Foucher, L.; Barroca, M.J.; Dulyanska, Y.; Correia, P.M.R.; Guiné, R.P.F. Development of Innovative Candied Chestnuts from Three Chestnut Cultivars Grown in Portugal. Foods 2022, 11, 917. [Google Scholar] [CrossRef]
- Diamandis, S. Sweet chestnut (Castanea sativa): A nut tree with great potential still to be exploited. Acta Hortic. 2008, 784, 37–42. [Google Scholar] [CrossRef]
- Moura, A. Seleção clonal na variedade de castanha “Longal” visando a sua aptidão agronómica e agroalimentar. Ph.D. Thesis, Universidade de Tras-os-Montes e Alto Douro, Vila Real, Portugal, 2018. [Google Scholar]
- Ertan, E.; Erdal, E.; Alkan, G.; Algül, B.E. Effects of different postharvest storage methods on the quality parameters of chestnuts (Castanea sativa Mill.). HortScience 2015, 50, 577–581. [Google Scholar] [CrossRef]
- Mota, M.; Marques, T.; Pinto, T.; Raimundo, F.; Borges, A.; Caço, J.; Gomes-Laranjo, J. Relating plant and soil water content to encourage smart watering in chestnut trees. Agric. Water Manag. 2018, 203, 30–36. [Google Scholar] [CrossRef]
- Carneiro-Carvalho, A.; Aires, A.; Anjos, R.; Martins, L.; Pinto, T.; Peixoto, F.; Gomes-Laranjo, J. The role of silicon fertilization in the synthesis of phenolic compounds on chestnut plants infected with P. cinnamomi and C. parasitica. J. Plant Dis. Prot. 2020, 127, 211–227. [Google Scholar] [CrossRef]
- Eurostat. Data of Crop of Chestnut Production in EU Standard Humidity. 2021. Available online: https://www.europa.eu (accessed on 6 September 2022).
- Suna, S.; Avşar, B.; Koçer, S.; Çopur, Ö.U. Effects of different pretreatments on the physicochemical characteristics and quality criteria of chestnut (Castanea sativa Mill.) pickle: A new value-added product. J. Food Process. Preserv. 2021, 45, e15669. [Google Scholar] [CrossRef]
- Cevriye, M.E.R.T.; Ertürk, Ü. Chemical compositions and sugar profiles of consumed chestnut cultivars in the Marmara Region, Turkey. Not. Bot. Horti Agrobot. 2017, 45, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.; Pinto, T.; Vilela, A.; Marques, T.; Borges, A.; Caço, J.; Ferreira-Cardoso, J.; Raimundo, F.; Gomes-Laranjo, J. Irrigation positively affects the chestnut’s quality: The chemical composition, fruit size and sensory attributes. Sci. Hortic. 2018, 238, 177–186. [Google Scholar] [CrossRef]
- Delgado, T.; Pereira, J.A.; Casal, S.; Ramalhosa, E. Chapter 6: Bioactive Compounds of Chestnuts as Health Promoters. In Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters Part II; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; Volume 6, pp. 132–154. ISBN 978-1-68108-244-8. [Google Scholar]
- Corona, P.; Frangipane, M.T.; Moscetti, R.; Feudo, G.L.; Castellotti, T.; Massantini, R. Chestnut cultivar identification through the data fusion of sensory quality and ft-nir spectral data. Foods 2021, 10, 2575. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Pereira, J.A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Sugars profiles of different chestnut (Castanea sativa Mill.) and almond (Prunus dulcis) cultivars by HPLC-RI. Plant Foods Hum. Nutr. 2010, 65, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Poljak, I.; Vahčić, N.; Vidaković, A.; Tumpa, K.; Žarković, I.; Idžojtić, M. Traditional sweet chestnut and hybrid varieties: Chemical composition, morphometric and qualitative nut characteristics. Agronomy 2021, 11, 516. [Google Scholar] [CrossRef]
- Míguez Bernárdez, M.M.; De la Montaña Miguélez, J.; García Queijeiro, J. HPLC determination of sugars in varieties of chestnut fruits from Galicia (Spain). J. Food Compos. Anal. 2004, 17, 63–67. [Google Scholar] [CrossRef]
- Cristofori, V.; Muganu, M.; Graziosi, P.; Bertazza, G.; Bignami, C. Comparison of nut traits and quality evaluation of chestnut (Castanea sativa Mill.) germplasm in Latium Region (Central Italy). Acta Hortic. 2009, 815, 133–140. [Google Scholar] [CrossRef]
- Silva, A.P.; Oliveira, I.; Silva, M.E.; Guedes, C.M.; Borges, O.; Magalhães, B.; Gonçalves, B. Starch characterization in seven raw, boiled and roasted chestnuts (Castanea sativa Mill.) cultivars from Portugal. J. Food Sci. Technol. 2016, 53, 348–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, O.; Gonçalves, B.; De Carvalho, J.L.S.; Correia, P.; Silva, A.P. Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chem. 2008, 106, 976–984. [Google Scholar] [CrossRef]
- Massantini, R.; Moscetti, R.; Frangipane, M.T. Evaluating progress of chestnut quality: A review of recent developments. Food Sci. Technol. 2021, 113, 245–254. [Google Scholar] [CrossRef]
- NDB Number:12098. National Nutrient Database for Standard Reference. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA: Beltsville, MD, USA. 2016. Available online: https://www.usda.gov (accessed on 28 May 2022).
- Gonçalves, B.; Borges, O.; Costa, H.S.; Bennett, R.; Santos, M.; Silva, A.P. Metabolite composition of chestnut (Castanea sativa Mill.) upon cooking: Proximate analysis, fibre, organic acids and phenolics. Food Chem. 2010, 122, 154–160. [Google Scholar] [CrossRef]
- Rodrigues, P.; Ferreira, T.; Nascimento-Gonçalves, E.; Seixas, F.; da Costa, R.M.G.; Martins, T.; Neuparth, M.J.; Pires, M.J.; Lanzarin, G.; Félix, L.; et al. Dietary supplementation with chestnut (Castanea sativa) reduces abdominal adiposity in FVB/n mice: A preliminary study. Biomedicines 2020, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) burs extracts and functional compounds: UHPLC-UV-HRMS profiling, antioxidant activity, and inhibitory effects on phytopathogenic fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [Green Version]
- Choupina, A.B. Nutritional and health potential of European chestnut. Rev. Ciências Agrárias 2019, 42, 801–807. [Google Scholar]
- Pinto, D.; Rodrigues, F.; Braga, N.; Santos, J.; Pimentel, F.B.; Palmeira-De-Oliveira, A.; Oliveira, M.B.P.P. The Castanea sativa bur as a new potential ingredient for nutraceutical and cosmetic outcomes: Preliminary studies. Food Funct. 2016, 8, 201–208. [Google Scholar] [CrossRef]
- Brochard, M.; Correia, P.; Barroca, M.J.; Guiné, R.P.F. Development of a new pasta product by the incorporation of chestnut flour and bee pollen. Appl. Sci. 2021, 11, 6617. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Abouelenein, D.; Acquaticci, L.; Alessandroni, L.; Abd-Allah, R.H.; Borsetta, G.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. Effect of roasting, boiling, and frying processing on 29 polyphenolics and antioxidant activity in seeds and shells of sweet chestnut (Castanea sativa Mill.). Plants 2021, 10, 2192. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Nickel, A.; Kuntz, S.; Daniel, H. Ascorbic acid suppresses drug-induced apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. Carcinogenesis 2004, 25, 703–712. [Google Scholar] [CrossRef] [Green Version]
- FAO; World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; pp. 1–20. ISBN 92-4-154612-3. [Google Scholar]
- Karasek, L.; Wenzl, T.; Anklam, E. Determination of acrylamide in roasted chestnuts and chestnut-based foods by isotope dilution HPLC-MS/MS. Food Chem. 2009, 114, 1555–1558. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Squillaci, G.; D’Apolito, M.; Petillo, O.; Veraldi, F.; Cara, F.L.; Peluso, G.; Margarucci, S.; Morana, A. Castanea sativa Mill. shells aqueous extract exhibits anticancer properties inducing cytotoxic and pro-apoptotic effects. Molecules 2019, 24, 3401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, V.; Ferreira, M.M. O Vinho Sentido—Sem Descrever Aromas ou Atribuir Pontuações; Plátano Editora: Lisbon, Portugal, 2019; p. 224. ISBN 978-989-760-255-9. [Google Scholar]
- Precone, V.; Beccari, T.; Stuppia, L.; Baglivo, M.; Paolacci, S.; Manara, E.; Miggiano, G.A.D.; Falsini, B.; Trifirò, A.; Zanlari, A.; et al. Taste, olfactory and texture related genes and food choices: Implications on health status. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1305–1321. [Google Scholar] [CrossRef]
- ISO, 5492; International Organization for Standardization (ISO)—Sensory analysis—Vocabulary. ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38051.html (accessed on 28 May 2022).
- Almeida, A. Training taste: Early markers of healthy eating for life. Monografia. Faculdade de Ciências da Nutrição da Universidade do Porto. 2010. Available online: http://hdl.handle.net/10216/54777 (accessed on 20 May 2022).
- Beauchamp, G.K.; Mennella, J.A. Flavor perception in human infants: Development and functional significance. Digestion 2011, 83, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Canon, F.; Neiers, F.; Guichard, E. Saliva and Flavor Perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879. [Google Scholar] [CrossRef] [PubMed]
- Motoi, L.; Morgenstern, M.P.; Paredes, D.; Wilson, A.J.; Hedderley, D.I.; Wade, C.; Tartaglia, J.M.; Green, C. The effect of flavour modulators on chewing gum flavour duration. Int. J. Food Sci. Technol. 2019, 54, 3119–3127. [Google Scholar] [CrossRef]
- Kim, U.K.; Breslin, P.A.S.; Reed, D.; Drayna, D. Genetics of human taste perception. J. Dent. Res. 2004, 83, 448–453. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Tasting—A Professional Handbook; Academic Press: London, UK, 2009; ISBN 978-0-12-374181-3. [Google Scholar]
- Ervina, E.; Berget, I.; Almli, L. Sensitivities, Fattiness Sensitivity, and Food Liking in 11-Year-Old Children. Foods 2020, 9, 1315. [Google Scholar] [CrossRef]
- Ceolin, J.; Pinheiro, T.D.L.F. Sensibilidade gustativa em idosos: Uma revisão narrativa. PAJAR—Pan Am. J. Aging Res. 2017, 5, 78. [Google Scholar] [CrossRef]
- Sanematsu, K.; Yoshida, R.; Shigemura, N.; Ninomiya, Y. Structure, function, and signaling of taste G-protein-coupled receptors. Curr. Pharm. Biotechnol. 2014, 15, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Dalziel, J.E. G-Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020, 11, 587664. [Google Scholar] [CrossRef] [PubMed]
- Von Molitor, E.; Riedel, K.; Hafner, M.; Rudolf, R.; Cesetti, T. Sensing senses: Optical biosensors to study gustation. Sensors 2020, 20, 1811. [Google Scholar] [CrossRef] [Green Version]
- Bigiani, A. The origin of saltiness: Oral detection of NaCl. Curr. Opin. Physiol. 2021, 19, 156–161. [Google Scholar] [CrossRef]
- Liman, E.R. Salty Taste: From Transduction to Transmitter Release, Hold the Calcium. Neuron 2020, 106, 709–711. [Google Scholar] [CrossRef]
- Bigiani, A. Salt Taste. In The Senses: A Comprehensive Reference, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 247–263. [Google Scholar] [CrossRef]
- Lutfi, Z.; Alam, F.; Nawab, A.; Haq, A.; Hasnain, A. Effect of NaCl on physicochemical properties of xanthan gum—Water chestnut starch complexes. Int. J. Biol. Macromol. 2019, 131, 557–563. [Google Scholar] [CrossRef]
- Lutfi, Z.; Nawab, A.; Alam, F.; Reza, M.; Hasnain, A. Effects of Ionic Compounds on Optimization of Physicochemical Properties of Biopolymer Isolated from Pakistani Water Chestnuts. Int. J. Biotechnol. 2019, 16, 607–612. [Google Scholar]
- Hanukoglu, I. ASIC and ENaC type sodium channels: Conformational states and the structures of the ion selectivity filters. FEBS J. 2017, 284, 525–545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jin, H.; Zhang, W.; Ding, C.; O’Keeffe, S.; Ye, M.; Zuker, C.S. Sour Sensing from the Tongue to the Brain. Cell 2019, 179, 392–402. [Google Scholar] [CrossRef]
- Liman, E.R.; Kinnamon, S.C. Sour taste: Receptors, cells and circuits. Curr. Opin. Physiol. 2021, 20, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Briand, L.; Salles, C. Taste perception and integration. In Flavor: From Food to Behaviors, Wellbeing and Health; Woodhead Publishing: Cambridge, UK, 2016; pp. 101–119. [Google Scholar] [CrossRef]
- Lee, A.A.; Owyang, C. Sugars, Sweet Taste Receptors, and Brain Responses. Nutrients 2017, 9, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milinovic, J.; Mata, P.; Diniz, M.; Noronha, J.P. Umami taste in edible seaweeds: The current comprehension and perception. Int. J. Gastron. Food Sci. 2021, 23, 100301. [Google Scholar] [CrossRef]
- Magalhães, G.M.F. Mecanismos Bioquímicos da Percepção do Doce e Preferências Alimentares. Master’s Thesis, Faculdade de Farmácia da Universidade do Porto, Porto, Portugal, 2013. [Google Scholar]
- Han, P.; Bagenna, B.; Fu, M. The sweet taste signalling pathways in the oral cavity and the gastrointestinal tract affect human appetite and food intake: A review. Int. J. Food Sci. Nutr. 2019, 70, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, S.C. Umami taste transduction mechanisms. Am. J. Clin. Nutr. 2009, 90, 753–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Liu, Z. Receptor, signal transduction and evolution of sweet, umami and bitter taste. Mar. Life Sci. Technol. 2020, 2, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Servant, G.; Kenakin, T.; Zhang, L.; Williams, M.; Servant, N. The function and allosteric control of the human sweet taste receptor. Adv. Pharmacol. 2020, 88, 59–82. [Google Scholar] [CrossRef]
- Dang, Y.; Hao, L.; Cao, J.; Sun, Y.; Zeng, X.; Wu, Z.; Pan, D. Molecular docking and simulation of the synergistic effect between umami peptides, monosodium glutamate and taste receptor T1R1/T1R3. Food Chem. 2019, 271, 697–706. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Liu, Y. Characterization and evaluation of umami taste: A review. TrAC—Trends Anal. Chem. 2020, 127, 115876. [Google Scholar] [CrossRef]
- Miranda, A.M.; Ingram, M.; Nuessle, T.M.; Santorico, S.A.; Garneau, N.L. Factors affecting detection of a bimodal sour-savory mixture and inter-individual umami taste perception. Food Qual. Prefer. 2021, 89, 104147. [Google Scholar] [CrossRef]
- Maina, I.W.; Workman, A.D.; Cohen, N.A. The role of bitter and sweet taste receptors in upper airway innate immunity: Recent advances and future directions. World J. Otorhinolaryngol.—Head Neck Surg. 2018, 4, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Breslin, P.A.S.; Huang, L. Human taste: Peripheral anatomy, taste transduction, and coding. Adv. Oto-Rhino-Laryngol. 2006, 63, 152–190. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, C.H.; Lifshitz, L.M.; ZhuGe, R. Extraoral bitter taste receptors in health and disease. J. Gen. Physiol. 2017, 149, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Grassin-Delyle, S.; Salvator, H.; Mantov, N.; Abrial, C.; Brollo, M.; Faisy, C.; Naline, E.; Couderc, L.J.; Devillier, P. Bitter Taste Receptors (TAS2Rs) in Human Lung Macrophages: Receptor Expression and Inhibitory Effects of TAS2R Agonists. Front. Physiol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Hayes, J.E. Regional variation of bitter taste and aftertaste in humans. Chem. Senses 2019, 44, 721–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaggiari, G.; Di Pizio, A.; Cozzini, P. Sweet, umami and bitter taste receptors: State of the art of in silico molecular modeling approaches. Trends Food Sci. Technol. 2020, 96, 21–29. [Google Scholar] [CrossRef]
- Hu, M.; Yang, X.; Chang, X. Bioactive phenolic components and potential health effects of chestnut shell: A review. J. Food Biochem. 2021, 45, e13696. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhang, J.; Yan, L.; Liu, S.; Taha, A.A.; Wang, J.; Ma, C. Subcritical water extraction of phenolic antioxidants with improved α-amylase and α-glucosidase inhibitory activities from exocarps of Castanea mollissima Blume. J. Supercrit. Fluids 2020, 158, 104747. [Google Scholar] [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Vella, F.M.; De Masi, L.; Calandrelli, R.; Morana, A.; Laratta, B. Valorization of the agro-forestry wastes from Italian chestnut cultivars for the recovery of bioactive compounds. Eur. Food Res. Technol. 2019, 245, 2679–2686. [Google Scholar] [CrossRef]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the Phenolic Profile of Castanea sativa Mill. By-Products and Their Antioxidant and Antimicrobial Activity against Multiresistant Bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, L.P.; Keast, R.S.J. The test-retest reliability of fatty acid taste thresholds. Chemosens. Percept. 2013, 6, 70–77. [Google Scholar] [CrossRef]
- Simons, P.J.; Kummer, J.A.; Luiken, J.J.F.P.; Boon, L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 2011, 113, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, T.A.; Khan, N.A. Cell signaling mechanisms of oro-gustatory detection of dietary fat: Advances and challenges. Prog. Lipid Res. 2014, 53, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Morini, G.; Maga, J.A. Changes in the fatty acid composition of roasted and boiled Chinese (Castanea molissima) and Italian (C. sativa) chestnuts grown at the same location. Dev. Food Sci. 1995, 37, 563–568. [Google Scholar] [CrossRef]
- Laguna, L.; Bartolomé, B.; Moreno-Arribas, M.V. Mouthfeel perception of wine: Oral physiology, components and instrumental characterization. Trends Food Sci. Technol. 2017, 59, 49–59. [Google Scholar] [CrossRef]
- Norton, V.; Lignou, S.; Methven, L. Influence of age and individual differences on mouthfeel perception of whey protein-fortified products: A review. Foods 2021, 10, 433. [Google Scholar] [CrossRef]
- Simons, C.T.; Klein, A.H.; Carstens, E. Chemogenic Subqualities of Mouthfeel. Chem. Senses 2019, 44, 281–288. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; Lucia, A.D.; Colucci, G.; Cara, F.L.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Mujić, I.; Zivković, J.; Savić, V.; Alibabić, V.; Staver, M.; Jug, T.; Franić, M.; Damijanić, K. Analysis of volatile compounds in chestnut using solid-phase microextraction coupled with GC-MS. Acta Hortic. 2018, 1220, 203–207. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Ennis, M.; Puche, A.C.; Holy, T.; Shipley, M.T. Chapter 29—The Olfactory System. In The Rat Nervous System, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 923–964. [Google Scholar] [CrossRef]
- Molnar, C.; Gair, J. Concepts of Biology, 1st ed.; BCCampus: Victoria, BC, Canada, 2019. [Google Scholar]
- Dalesio, N.M.; Barreto Ortiz, S.F.; Pluznick, J.L.; Berkowitz, D.E. Olfactory, taste, and photo sensory receptors in non-sensory organs: It just makes sense. Front. Physiol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krist, S.; Unterweger, H.; Bandion, F.; Buchbauer, G. Volatile compound analysis of SPME headspace and extract samples from roasted Italian chestnuts (Castanea sativa Mill.) using GC-MS. Eur. Food Res. Technol. 2004, 219, 470–473. [Google Scholar] [CrossRef]
- Marques, C.; Correia, E.; Dinis, L.-T.; Vilela, A. An Overview of Sensory Characterization Techniques: Profiling Methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef]
- Künsch, U.; Schärer, H.; Patrian, B.; Hurter, J.; Conedera, M.; Sassella, A.; Jermini, M.; Jelmini, G. Quality assessment of chestnut fruits. Acta Hortic. 1999, 494, 119–127. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; de Torres, C.; Pérez-Coello, M.S. Effect of geographical origin on the chemical and sensory characteristics of chestnut honeys. Food Res. Intern. 2010, 43, 2335–2340. [Google Scholar] [CrossRef]
- Carneiro-Carvalho, A.; Vilela, A.; Ferreira-Cardoso, J.; Marques, T.; Anjos, R.; Gomes-Laranjo, J.; Pinto, T. Productivity, chemical composition and sensory quality of “Martaínha” chestnut variety treated with Silicon. CYTA—J. Food 2019, 17, 316–323. [Google Scholar] [CrossRef]
- ISO 4121; Sensory Analysis—Guidelines for the Use of Quantitative Response Scales. International Organization of Standardization: London, UK, 2003. Available online: http://www.iso.org/iso/home/store/catalogue_ics/catalogue_detail_ics.htm?ics1=67&ics2=240&ics3=&csnumber=33817 (accessed on 20 May 2022).
- Biancolillo, A.; De Luca, S.; Bassi, S.; Roudier, L.; Bucci, R.; Magrì, A.D.; Marini, F. Authentication of an Italian PDO hazelnut (“Nocciola Romana”) by NIR spectroscopy. Environ. Sci. Pollut. Res. 2018, 25, 28780–28786. [Google Scholar] [CrossRef]
- Chapman, J.; Elbourne, A.; Truong, V.K.; Newman, L.; Gangadoo, S.; Rajapaksha Pathirannahalage, P.; Cheeseman, S.; Cozzolino, D. Sensomics—From conventional to functional NIR spectroscopy—Shining light over the aroma and taste of foods. Trends Food Sci. Technol. 2019, 91, 274–281. [Google Scholar] [CrossRef]
- Jie, L.; Xiaoyu, L.; Wei, W.; Wu, X.; Jun, Z.; Zhu, Z. Measurement of protein content in chestnuts using near infrared spectroscopy. J. Chem. Pharm. Res. 2014, 6, 938–941. [Google Scholar]
- Liu, J.; Li, X.; Li, P.; Wang, W.; Zhang, J.; Zhou, W.; Zhou, Z. Non-destructive measurement of sugar content in chestnuts using near-infrared spectroscopy. IFIP Adv. Inf. Commun. Technol. 2011, 347, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Gounga, M.E.; Xu, S.Y.; Wang, Z. Nutritional and microbiological evaluations of chocolate-coated Chinese chestnut (Castanea mollissima) fruit for commercial use. J. Zhejiang Univ. Sci. B 2008, 9, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Moreira, R.; Chenlo, F.; Chaguri, L.; Vázquez, G. Air drying and colour characteristics of chestnuts pre-submitted to osmotic dehydration with sodium chloride. Food Bioprod. Process. 2011, 89, 109–115. [Google Scholar] [CrossRef]
- Comba, L.; Gay, P.; Piccarolo, P.; Ricauda Aimonino, D. Thermal processes in the candy process of chestnut. Acta Hortic. 2010, 866, 587–594. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Liu, W.; Jia, X.; Wang, S.; Qiao, X.; Cheng, X. Antioxidant activity of pickled sauced meat before and after cooking and in vitro gastrointestinal digestion. J. Food Process. Preserv. 2021, 45, e14922. [Google Scholar] [CrossRef]




| Nutrient Value | |||
|---|---|---|---|
| Raw | Roasted | Boiled | |
| Energy (Kcal) | 196 | 245 | 131 |
| General composition (g) | |||
| Water | 52 | 40.5 | 68.2 |
| Protein | 1.63 | 3.17 | 2 |
| Total lipid (fat) | 1.25 | 2.2 | 1.38 |
| Fatty acids, total saturated | 0.235 | 0.414 | 0.26 |
| Fatty acids, total monounsaturated | 0.43 | 0.759 | 0.476 |
| Fatty acids, total polyunsaturated | 0.493 | 0.869 | 0.545 |
| Carbohydrates | 44.2 | 53 | 27.8 |
| Fiber, total dietary | 5.1 | ||
| Sugars, total including NLEA 1 | 10.6 | ||
| Vitamins | |||
| Folates (µg) | 58 | 70 | 38 |
| Niacin (mg) | 1.1 | 1.34 | 0.731 |
| Pantothenic acid (mg) | 0.476 | 0.554 | 0.316 |
| Riboflavin (mg) | 0.016 | 0.175 | 0.104 |
| Thiamin (mg) | 0.144 | 0.243 | 0.148 |
| Vitamin A (IU) | 26 | 24 | 17 |
| Vitamin C (mg) | 40.2 | 26 | 26.7 |
| Electrolytes (mg) | |||
| Sodium | 2 | 2 | 27 |
| Potassium | 484 | 592 | 715 |
| Minerals (mg) | |||
| Calcium | 19 | 29 | 46 |
| Cooper | 0.418 | 0.507 | 0.472 |
| Iron, Fe | 0.94 | 0.91 | 1.73 |
| Magnesium | 30 | 33 | 54 |
| Manganese | 0.336 | 1.18 | 0.854 |
| Phosphorus | 38 | 107 | 99 |
| Zinc | 0.49 | 0.57 | 0.25 |
| Compound Name | Flavor Description | Concentration Calculated by Peak Area (%) | ||
|---|---|---|---|---|
| Peeled Chestnuts | Unpeeled Chestnuts | |||
| Alcohols | ||||
| Ethanol | Alcoholic | 20.61 ± 1.02 | 14.19 ± 2.57 | |
| 2-Propanol | Butter | ND | 1.64 ± 0.57 | |
| 1-Propanol | Alcohol, apple, musty, fruity, peanut, pear | ND | 0.34 ± 0.08 | |
| 2-Methyl-1-propanol | Fruity, Wine-like | ND | 3.57 ± 0.31 | |
| 2-Pentanol | Oily, green | ND | 0.76 ± 0.19 | |
| 3-Methyl-1-butanol | Oily, whiskey | ND | 5.27 ± 0.83 | |
| 2-Methyl-1-butanol | Fuel oil, sweet | 0.75 ± 0.13 | 2.86 ± 0.59 | |
| 1-Pentanol | Sweet, vanilla | 1.54 ± 0.26 | 0.55 ± 0.09 | |
| 2,3-Butanediol | Neutral sensory characteristics | 2.46 ± 0.11 | ND | |
| 1-Hexanol | Green, herbaceous, woody | 6.90 ± 1.38 | 2.82 ± 0.56 | |
| 2-Heptanol | Oily, earthy | 0.76 ± 0.19 | 0.69 ± 0.16 | |
| 1-Octen-3-ol | Cheese, creamy, earthy, herbaceous | ND | 2.32 ± 0.45 | |
| 3-Octanol | Melon, musty, oily | 0.54 ± 0.13 | 5.02 ± 0.23 | |
| 1-Octanol | Citrus, fatty, woody | 18.40 ± 4.05 | 13.06 ± 1.72 | |
| Phenylethyl alcohol | Honey, rose | 0.74 ± 0.19 | 0.91 ± 0.41 | |
| Total alcohols | 52.80 ± 4.66 | 54.00 ± 2.31 | ||
| Aldehydes | ||||
| Octanal | Honey, fruity, fatty, citrus | 0.61 ± 0.22 | ND | |
| Benzaldehyde | Almond, cherry, sweet | 0.58 ± 0.19 | 0.41 ± 0.10 | |
| Total aldehydes | 1.19 ± 0.36 | 0.41 ± 0.10 | ||
| Ketones | ||||
| Acetone | Apple, ethereal | 2.97 ± 1.01 | 1.45 ± 0.27 | |
| 2-Pentanone | Alcohol, apples, banana, cheese | 0.87 ± 0.19 | 1.35 ± 0.27 | |
| 2,3-Pentanedione | Buttery, cheesy, sweet, nutty, fruity | 0.72 ± 0.09 | 0.62 ± 0.17 | |
| 3-Hydroxy-2-butanone | Sweet, buttery, creamy, dairy | ND | 0.87 ± 0.09 | |
| 2-Heptanone | Banana, cinnamon, spicy, fruity | 5.86 ± 1.14 | 2.37 ± 0.49 | |
| 2-Octanone | Green, herbaceous, floral, fruity | 0.91 ± 0.18 | ND | |
| 6-Methyl-5-hepten-2-one | Oily, herbaceous, green | 0.60 ± 0.09 | ND | |
| Total ketones | 11.93 ± 2.03 | 6.66 ± 0.44 | ||
| Esters | ||||
| Methyl acetate | Ethereal, sweet | 1.27 ± 0.29 | 0.45 ± 0.08 | |
| Ethyl 2-methyl propanoate | Sharp, sweet, green, apple, fruity | ND | 0.64 ± 0.14 | |
| Ethyl butyrate | Tropical fruit, tutti-fruity, mango flavor | 0.84 ± 0.07 | 0.45 ± 0.05 | |
| 3-Methylbutyl acetate | Fruity, pear, banana-like odor | 0.75 ± 0.10 | ND | |
| 2-Methylbutyl acetate | Banana, peanut | 0.72 ± 0.06 | 0.72 ± 0.16 | |
| Methyl octanoate | Fruity, green, citrus | ND | 2.95 ± 0.39 | |
| Octyl acetate | Jasmine, herbaceous, fruity | 1.01 ± 0.22 | 0.38 ± 0.06 | |
| Total esters | 4.59 ± 0.35 | 5.59 ± 0.77 | ||
| Terpenoids | ||||
| Limonene | Lemon, orange, citrus, sweet | 1.73 ± 0.21 | 0.84 ± 0.46 | |
| Total terpenoids | 1.73 ± 0.48 | 0.84 ± 0.24 | ||
| Acids | ||||
| Acetic acid | Strong, pungent sour odor | 11.01 ± 2.10 | 4.28 ± 0.85 | |
| Hexanoic acid | Cheese, fatty, sour | 1.39 ± 0.34 | ND | |
| Total acids | 12.40 ± 2.57 | 4.28 ± 0.93 | ||
| Furans | ||||
| Tetrahydrofuran | Ether-like | 0.57 ± 0.07 | ND | |
| 2-Pentylfuran | Green bean, metallic, vegetable | 10.26 ± 0.26 | 2.02 ± 0.55 | |
| Total furans | 10.83 ± 1.39 | 2.02 ± 0.41 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.J.; Pinto, T.; Vilela, A. Sweet Chestnut (Castanea sativa Mill.) Nutritional and Phenolic Composition Interactions with Chestnut Flavor Physiology. Foods 2022, 11, 4052. https://doi.org/10.3390/foods11244052
Santos MJ, Pinto T, Vilela A. Sweet Chestnut (Castanea sativa Mill.) Nutritional and Phenolic Composition Interactions with Chestnut Flavor Physiology. Foods. 2022; 11(24):4052. https://doi.org/10.3390/foods11244052
Chicago/Turabian StyleSantos, Maria João, Teresa Pinto, and Alice Vilela. 2022. "Sweet Chestnut (Castanea sativa Mill.) Nutritional and Phenolic Composition Interactions with Chestnut Flavor Physiology" Foods 11, no. 24: 4052. https://doi.org/10.3390/foods11244052
APA StyleSantos, M. J., Pinto, T., & Vilela, A. (2022). Sweet Chestnut (Castanea sativa Mill.) Nutritional and Phenolic Composition Interactions with Chestnut Flavor Physiology. Foods, 11(24), 4052. https://doi.org/10.3390/foods11244052