Tocochromanol Profiles in Chlorella sorokiniana, Nannochloropsis limnetica and Tetraselmis suecica Confirm the Presence of 11′-α-Tocomonoenol in Cultured Microalgae Independently of Species and Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Samples, Culture Conditions, and Sample Pretreatment
2.2. Standards
2.3. Tocochromanol Extraction for Liquid Chromatography
2.4. Identification of α-Tocomonoenol by Liquid Chromatography Coupled with Mass Spectrometry (LC-MSn)
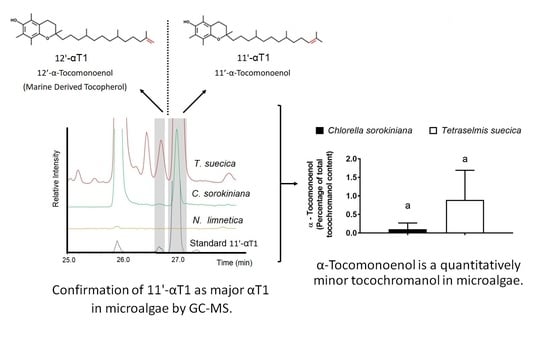
2.5. Identification of α-Tocomonoenol by Gas Chromatography–Mass Spectrometry (GC-MS)
2.6. Quantification of Tocochromanols by HPLC-FLD
2.7. Determination of Fatty Acids as Methyl Esters (FAME) by GC-MS
2.8. Statistical Analysis
3. Results and Discussion
3.1. Identification of 11′-αT1 and Quantification of Tocochromanols
3.2. Fatty Acid Profiles and Correlations of Fatty Acids with Tocochromanols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merlo, S.; Gabarrell Durany, X.; Pedroso Tonon, A.; Rossi, S. Marine Microalgae Contribution to Sustainable Development. Water 2021, 13, 1373. [Google Scholar] [CrossRef]
- Tocher, D.; Betancor, M.; Sprague, M.; Olsen, R.; Napier, J. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Benemann, J.R.; Zhang, X.; Hu, H.; Qin, S. Microalgal industry in China: Challenges and prospects. J. Appl. Phycol. 2016, 28, 715–725. [Google Scholar] [CrossRef]
- Yamaguchi, K. Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass and metabolites: A review. J. Appl. Phycol. 1996, 8, 487–502. [Google Scholar] [CrossRef]
- Lafarga, T. Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Rev. Int. 2020, 36, 559–583. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.-Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Diprat, A.B.; Silveira Thys, R.C.; Rodrigues, E.; Rech, R. Chlorella sorokiniana: A new alternative source of carotenoids and proteins for gluten-free bread. LWT 2020, 134, 109974. [Google Scholar] [CrossRef]
- Chen, W.; Luo, L.; Han, D.; Long, F.; Chi, Q.; Hu, Q. Effect of dietary supplementation with Chlorella sorokiniana meal on the growth performance, antioxidant status, and immune response of rainbow trout (Oncorhynchus mykiss). J. Appl. Phycol. 2021, 33, 3113–3122. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Jesisca; Hsieh, C.; Lee, D.-J.; Chang, C.-H.; Chang, J.-S. Production, extraction and stabilization of lutein from microalga Chlorella sorokiniana MB-1. Bioresour. Technol. 2016, 200, 500–505. [Google Scholar] [CrossRef]
- Gómez-Jacinto, V.; Navarro-Roldán, F.; Garbayo-Nores, I.; Vílchez-Lobato, C.; Borrego, A.A.; García-Barrera, T. In vitro selenium bioaccessibility combined with in vivo bioavailability and bioactivity in Se-enriched microalga (Chlorella sorokiniana) to be used as functional food. J. Funct. Foods 2020, 66, 103817. [Google Scholar] [CrossRef]
- Freire, I.; Cortina-Burgueño, A.; Grille, P.; Arizcun Arizcun, M.; Abellán, E.; Segura, M.; Witt Sousa, F.; Otero, A. Nannochloropsis limnetica: A freshwater microalga for marine aquaculture. Aquaculture 2016, 459, 124–130. [Google Scholar] [CrossRef]
- Marrez, D.A.; Cieślak, A.; Gawad, R.; Ebeid, H.M.; Chrenková, M.; Gao, M.; Yanza, Y.R.; El-Sherbiny, M.; Szumacher-Strabel, M. Effect of freshwater microalgae Nannochloropsis limnetica on the rumen fermentation in vitro. J. Anim. Feed Sci. 2017, 26, 359–364. [Google Scholar] [CrossRef]
- D’Souza, F.M.L.; Kelly, G.J. Effects of a diet of a nitrogen-limited alga (Tetraselmis suecica) on growth, survival and biochemical composition of tiger prawn (Penaeus semisulcatus) larvae. Aquaculture 2000, 181, 311–329. [Google Scholar] [CrossRef]
- Robert, R.; Parisi, G.; Rodolfi, L.; Poli, B.M.; Tredici, M.R. Use of fresh and preserved Tetraselmis suecica for feeding Crassostrea gigas larvae. Aquaculture 2001, 192, 333–346. [Google Scholar] [CrossRef]
- Birringer, M.; Siems, K.; Maxones, A.; Frank, J.; Lorkowski, S. Natural 6-hydroxy-chromanols and -chromenols: Structural diversity, biosynthetic pathways and health implications. RSC Adv. 2018, 8, 4803–4841. [Google Scholar] [CrossRef] [Green Version]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujisawa, A.; Hara, A.; Dunlap, W.C. An unusual vitamin E constituent (α-tocomonoenol) provides enhanced antioxidant protection in marine organisms adapted to cold-water environments. Proc. Natl. Acad. Sci. USA 2001, 98, 13144–13148. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Takahashi, S.; Nakano, K.; Kijima, S. Identification of New Vitamin E in Plant Oil. J. Japan Oil Chem. Soc. 1995, 44, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Beppu, F.; Aida, Y.; Kaneko, M.; Kasatani, S.; Aoki, Y.; Gotoh, N. Functional evaluation of marine-derived tocopherol, a minor homolog of vitamin E, on adipocyte differentiation and inflammation using 3T3-L1 and RAW264.7 cells. Fish. Sci. 2020, 86, 415–425. [Google Scholar] [CrossRef]
- Montoya-Arroyo, A.; Wagner, T.; Sus, N.; Müller, M.; Kröpfl, A.; Vetter, W.; Frank, J. Cytotoxicity, cellular uptake, and metabolism to short-chain metabolites of 11′-α-tocomonoenol is similar to RRR-α-tocopherol in HepG2 cells. Free Radic. Biol. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, A.; Dunlap, W.C.; Yamamoto, Y. Vitamin E protection in the biochemical adaptation of marine organisms to cold-water environments. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2010, 157, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, W.C.; Fujisawa, A.; Yamamoto, Y.; Moylan, T.J.; Sidell, B.D. Notothenioid fish, krill and phytoplankton from Antarctica contain a vitamin E constituent (α-tocomonoenol) functionally associated with cold-water adaptation. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2002, 133, 299–305. [Google Scholar] [CrossRef]
- Gotoh, N.; Mashimo, D.; Oka, T.; Sekiguchi, K.; Tange, M.; Watanabe, H.; Noguchi, N.; Wada, S. Analyses of marine-derived tocopherol in processed foods containing fish. Food Chem. 2011, 129, 279–283. [Google Scholar] [CrossRef]
- Ng, M.H.; Choo, Y.M.; Ma, A.N.; Chuah, C.H.; Hashim, M.A. Separation of vitamin E (tocopherol, tocotrienol, and tocomonoenol) in palm oil. Lipids 2004, 39, 1031–1035. [Google Scholar] [CrossRef]
- Butinar, B.; Bučar-Miklavčič, M.; Mariani, C.; Raspor, P. New vitamin E isomers (gamma-tocomonoenol and alpha-tocomonoenol) in seeds, roasted seeds and roasted seed oil from the Slovenian pumpkin variety ‘Slovenska golica’. Food Chem. 2011, 128, 505–512. [Google Scholar] [CrossRef]
- Irías-Mata, A.; Stuetz, W.; Sus, N.; Hammann, S.; Gralla, K.; Cordero-Solano, A.; Vetter, W.; Frank, J. Tocopherols, Tocomonoenols, and Tocotrienols in Oils of Costa Rican Palm Fruits: A Comparison between Six Varieties and Chemical versus Mechanical Extraction. J. Agric. Food Chem. 2017, 65, 7476–7482. [Google Scholar] [CrossRef]
- Hammann, S.; Englert, M.; Müller, M.; Vetter, W. Accelerated separation of GC-amenable lipid classes in plant oils by countercurrent chromatography in the co-current mode. Anal. Bioanal. Chem. 2015, 407, 9019–9028. [Google Scholar] [CrossRef]
- Müller, M.; Hammann, S.; Vetter, W. Countercurrent chromatographic isolation and purification of 11′-α-tocomonoenol from the vitamin E extract of palm oil. Food Chem. 2018, 256, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Mignogna, R.; Fratianni, A.; Niro, S.; Panfili, G. Tocopherol and tocotrienol analysis as a tool to discriminate different fat ingredients in bakery products. Food Control 2015, 54, 31–38. [Google Scholar] [CrossRef]
- Cain, N.; Alka, O.; Segelke, T.; von Wuthenau, K.; Kohlbacher, O.; Fischer, M. Food fingerprinting: Mass spectrometric determination of the cocoa shell content (Theobroma cacao L.) in cocoa products by HPLC-QTOF-MS. Food Chem. 2019, 298, 125013. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Arroyo, A.; Lehnert, K.; Lux, P.E.; Jiménez, V.M.; Esquivel, P.; Silva-Benavides, A.M.; Vetter, W.; Frank, J. 11′-α-Tocomonoenol is the major α-tocomonoenol isomer in cyanobacteria and microalgae from Costa Rica. J. Food Compos. Anal. 2022, 107, 104325. [Google Scholar] [CrossRef]
- Holdmann, C.; Schmid-Staiger, U.; Hornstein, H.; Hirth, T. Keeping the light energy constant—Cultivation of Chlorella sorokiniana at different specific light availabilities and different photoperiods. Algal Res. 2018, 29, 61–70. [Google Scholar] [CrossRef]
- Münkel, R.; Schmid-Staiger, U.; Werner, A.; Hirth, T. Optimization of outdoor cultivation in flat panel airlift reactors for lipid production by Chlorella vulgaris. Biotechnol. Bioeng. 2013, 110, 2882–2893. [Google Scholar] [CrossRef] [PubMed]
- Grebenstein, N.; Frank, J. Rapid baseline-separation of all eight tocopherols and tocotrienols by reversed-phase liquid-chromatography with a solid-core pentafluorophenyl column and their sensitive quantification in plasma and liver. J. Chromatogr. A 2012, 1243, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hammann, S.; Kröpfl, A.; Vetter, W. More than 170 polyunsaturated tocopherol-related compounds in a vitamin E capsule: Countercurrent chromatographic enrichment, gas chromatography/mass spectrometry analysis and preliminary identification of the potential artefacts. J. Chromatogr. A 2016, 1476, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Thurnhofer, S.; Vetter, W. A gas chromatography/electron ionization-mass spectrometry-selected ion monitoring method for determining the fatty acid pattern in food after formation of fatty acid methyl esters. J. Agric. Food Chem. 2005, 53, 8896–8903. [Google Scholar] [CrossRef] [PubMed]
- Wendlinger, C.; Hammann, S.; Vetter, W. Various concentrations of erucic acid in mustard oil and mustard. Food Chem. 2014, 153, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, N.; Watanabe, H.; Oka, T.; Mashimo, D.; Noguchi, N.; Hata, K.; Wada, S. Dietary Marine-Derived Tocopherol has a Higher Biological Availability in Mice Relative to Alpha-Tocopherol. Lipids 2009, 44, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bartosińska, E.; Buszewska-Forajta, M.; Siluk, D. GC–MS and LC–MS approaches for determination of tocopherols and tocotrienols in biological and food matrices. J. Pharm. Biomed. Anal. 2016, 127, 156–169. [Google Scholar] [CrossRef]
- Gee, P.T.; Liew, C.Y.; Thong, M.C.; Gay, M.C.L. Vitamin E analysis by ultra-performance convergence chromatography and structural elucidation of novel α-tocodienol by high-resolution mass spectrometry. Food Chem. 2016, 196, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Mudimu, O.; Koopmann, I.K.; Rybalka, N.; Friedl, T.; Schulz, R.; Bilger, W. Screening of microalgae and cyanobacteria strains for α-tocopherol content at different growth phases and the influence of nitrate reduction on α-tocopherol production. J. Appl. Phycol. 2017, 29, 2867–2875. [Google Scholar] [CrossRef]
- Safafar, H.; van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bong, S.C.; Loh, S.P. A study of fatty acid composition and tocopherol content of lipid extracted from marine microalgae, Nannochloropsis oculata and Tetraselmis suecica, using solvent extraction and supercritical fluid extraction. Int. Food Res. J. 2013, 20, 721–729. [Google Scholar]
- Carballo-Cárdenas, E.C.; Tuan, P.M.; Janssen, M.; Wijffels, R.H. Vitamin E (α-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol. Eng. 2003, 20, 139–147. [Google Scholar] [CrossRef]
- Goiris, K.; Van Colen, W.; Wilches, I.; León-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Santiago-Morales, I.S.; Trujillo-Valle, L.; Márquez-Rocha, F.J.; López Hernández, J.F. Tocopherols, phycocyanin and superoxide dismutase from microalgae: As potential food antioxidants. Appl. Food Biotechnol. 2018, 5, 19–27. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef]
- Fabregas, J.; Herrero, C. Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J. Ind. Microbiol. 1990, 5, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Yusof, Y.A.M.; Basari, J.M.H.; Mukti, N.A.; Sabuddin, R.; Muda, A.R.; Sulaiman, S.; Makpol, S.; Ngah, W.Z.W. Fatty acids composition of microalgae Chlorella vulgaris can be modulated by varying carbon dioxide concentration in outdoor culture. Afr. J. Biotechnol. 2011, 10, 13536–13542. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Maita, N.; Fujisawa, A.; Takashima, J.; Ishii, Y.; Dunlap, W.C. A new vitamin E (α-tocomonoenol) from eggs of the pacific salmon Oncorhynchus keta. J. Nat. Prod. 1999, 62, 1685–1687. [Google Scholar] [CrossRef]
- Hussain, N.; Irshad, F.; Jabeen, Z.; Shamsi, I.H.; Li, Z.; Jiang, L. Biosynthesis, Structural, and Functional Attributes of Tocopherols in Planta; Past, Present, and Future Perspectives. J. Agric. Food Chem. 2013, 61, 6137–6149. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H.K.; Niyogi, K.K. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ. 2005, 28, 1037–1045. [Google Scholar] [CrossRef]
- Schüler, L.M.; Schulze, P.S.C.; Pereira, H.; Barreira, L.; León, R.; Varela, J. Trends and strategies to enhance triacylglycerols and high-value compounds in microalgae. Algal Res. 2017, 25, 263–273. [Google Scholar] [CrossRef]




| Sample | RT (min) | Identified Ions |
|---|---|---|
| C. sorokiniana | 14.19 | m/z 429.3729 (C29H49O2; Δ ppm = 0.4) ([M + H]+) m/z 205.1226 (C13H17O2; Δ ppm = 1.6) m/z 165.0911 (C10H13O2; Δ ppm = 0.4) m/z 69.0706 (C5H9; Δ ppm = 11.0) |
| N. limnetica | 14.16 | m/z 429.3730 (C29H49O2; Δ ppm = 0.7) ([M + H]+) m/z 205.1224 (C13H17O2; Δ ppm = 0.4) m/z 165.0912 (C10H13O2; Δ ppm = 1.0) m/z 69.0707 (C5H9; Δ ppm = 11.3) |
| T. suecica | 14.17 | m/z 429.3727 (C29H49O2; Δ ppm = 0.1) ([M + H]+) m/z 205.1225 (C13H17O2; Δ ppm = 1.1) m/z 165.0911 (C10H13O2; Δ ppm = 0.6) m/z 69.0707 (C5H9; Δ ppm = 11.8) |
| Standard 11′-α-tocomonoenol | 14.24 | m/z 429.3734 (C29H49O2; Δ ppm = 1.6) ([M + H]+) m/z 205.1231 (C13H17O2; Δ ppm = 3.9) m/z 165.0913 (C10H13O2; Δ ppm = 1.5) m/z 69.0708 (C5H9; Δ ppm = 13.4) |
| Congener | Concentration (mg/kg DW) | ||
|---|---|---|---|
| Chlorella sorokiniana | Nannochloropsis limnetica | Tetraselmis suecica | |
| α-Tocopherol | 682 ± 9.0 a | 70.7 ± 23.03 b | 649 ± 86.3 a |
| β-Tocopherol | 0.64 ± 0.07 a | 0.23 ± 0.01 a | 4.4 ± 0.48 b |
| γ-Tocopherol | 0.05 ± 0.05 a | 0.11 ± 0.01 ab | 0.16 ± 0.01 b |
| δ-Tocopherol | 0.18 ± 0.02 | 0.22 ± 0.02 | 0.4 ± 0.41 |
| α-Tocomonoenol | 0.82 ± 1.43 | nd | 5.5 ± 4.83 |
| α-Tocotrienol | 37.4 ± 3.3 a | 10.5 ± 1.3 b | nd |
| β-Tocotrienol | 0.02 ± 0.00 a | 0.18 ± 0.04 b | nd |
| γ-Tocotrienol | 0.03 ± 0.01 a | 1.21 ± 0.09 b | nd |
| δ-Tocotrienol | nd | 0.06 ± 0.00 | nd |
| Total Tocopherols | 683 ± 112 a | 71.3 ± 23.1 b | 654 ± 85.8 a |
| Total Tocotrienols | 37.5 ± 3.3 a | 11.9 ± 1.4 b | nd |
| Total Tocochromanol | 721 ± 116a | 83.2 ± 24.4b | 659 ± 81.0a |
| Fatty Acid | Relative Fatty Acid Content (g/100 g FA) | ||
|---|---|---|---|
| Chlorella sorokiniana | Nannochloropsis limnetica | Tetraselmis suecica | |
| Monounsaturated | 12.7 ± 0.28 b | 18.6 ± 0.53 a | 20.4 ± 0.04 a |
| Di-unsaturated | 53.5 ± 0.31 a | 44.0 ± 0.64 b | 12.7 ± 0.10 c |
| Polyunsaturated | 17.0 ± 0.15 b | 11.7 ± 0.17 c | 40.8 ± 0.24 a |
| Total Unsaturated | 83.2 ± 0.18 a | 74.3 ± 0.26 b | 74.0 ± 0.24 b |
| Omega-3 | 17.0 ± 0.15 b | 11.7 ± 0.17 c | 25.2 ± 0.42 a |
| Omega-6 | 26.0 ± 0.13 b | 26.4 ± 0.05 a | 15.5 ± 0.09 c |
| Omega-9 | 5.4 ± 0.10 b | 6.0 ± 0.22 b | 10.7 ± 0.03 a |
| Main Variable | Secondary Variable (g/100 g FA) | Pearson Coefficient | p |
|---|---|---|---|
| Total tocochromanols (mg/kg DW) | Monounsaturated FA | −0.363 | 0.337 |
| Polyunsaturated FA | 0.554 | 0.121 | |
| Total unsaturated FA | 0.533 | 0.139 | |
| Omega-6 FA | −0.441 | 0.235 | |
| Omega-3 FA | 0.722 | 0.028 | |
| Relative tocopherols (%) | Monounsaturated FA | −0.049 | 0.899 |
| Polyunsaturated FA | 0.795 | 0.010 | |
| Total unsaturated FA | 0.223 | 0.564 | |
| Omega-6 FA | −0.712 | 0.031 | |
| Omega-3 FA | 0.902 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Arroyo, A.; Lehnert, K.; Muñoz-González, A.; Schmid-Staiger, U.; Vetter, W.; Frank, J. Tocochromanol Profiles in Chlorella sorokiniana, Nannochloropsis limnetica and Tetraselmis suecica Confirm the Presence of 11′-α-Tocomonoenol in Cultured Microalgae Independently of Species and Origin. Foods 2022, 11, 396. https://doi.org/10.3390/foods11030396
Montoya-Arroyo A, Lehnert K, Muñoz-González A, Schmid-Staiger U, Vetter W, Frank J. Tocochromanol Profiles in Chlorella sorokiniana, Nannochloropsis limnetica and Tetraselmis suecica Confirm the Presence of 11′-α-Tocomonoenol in Cultured Microalgae Independently of Species and Origin. Foods. 2022; 11(3):396. https://doi.org/10.3390/foods11030396
Chicago/Turabian StyleMontoya-Arroyo, Alexander, Katja Lehnert, Alejandra Muñoz-González, Ulrike Schmid-Staiger, Walter Vetter, and Jan Frank. 2022. "Tocochromanol Profiles in Chlorella sorokiniana, Nannochloropsis limnetica and Tetraselmis suecica Confirm the Presence of 11′-α-Tocomonoenol in Cultured Microalgae Independently of Species and Origin" Foods 11, no. 3: 396. https://doi.org/10.3390/foods11030396
APA StyleMontoya-Arroyo, A., Lehnert, K., Muñoz-González, A., Schmid-Staiger, U., Vetter, W., & Frank, J. (2022). Tocochromanol Profiles in Chlorella sorokiniana, Nannochloropsis limnetica and Tetraselmis suecica Confirm the Presence of 11′-α-Tocomonoenol in Cultured Microalgae Independently of Species and Origin. Foods, 11(3), 396. https://doi.org/10.3390/foods11030396