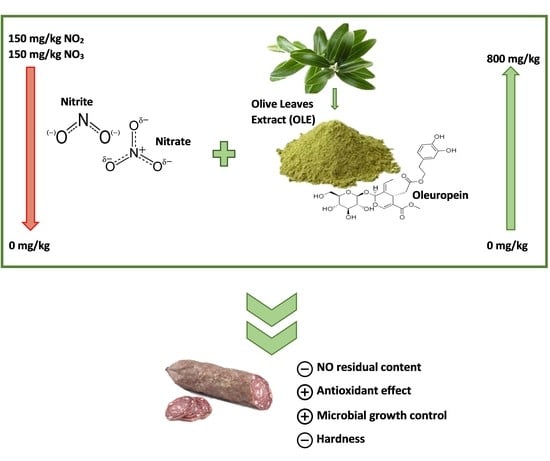
Olive Leaf Extract (OLE) Addition as Tool to Reduce Nitrate and Nitrite in Ripened Sausages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Characterization of Olive Leaf Extract (OLE)
2.2. Samples Preparation
2.3. Analytical Determination
2.3.1. Weight Loss, Water Activity (aw), pH, Moisture Content
2.3.2. Microbiological Analyses
2.3.3. Determination of Residual Nitrate and Nitrite
2.3.4. Colour Measurements
2.3.5. Determination of Texture Profile Analysis
2.3.6. Fatty Acid Determination
2.3.7. Test of Oxidation Stability
2.4. Statistical Analysis
3. Results
3.1. Characterisation of Olive Leaf Extract (OLE)
3.2. Microbiological Analysis, Nitrate and Nitrite Residual
3.3. Qualitative Characterization of Ripened Sausages
3.4. Colour Analysis
3.5. Texture Profile Analysis
3.6. Fatty Acids Composition
3.7. Test of Oxidation Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Summo, C.; De Angelis, D.; Difonzo, G.; Caponio, F.; Pasqualone, A. Effectiveness of Oat-Hull-based ingredient as fat replacer to produce low fat burger with high beta-glucans content. Foods 2020, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Coelho, D.; Blachier, F. Review of the association between meat consumption and risk of colorectal cancer. Nutr. Res. 2013, 33, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.H.; Du, H.Z.; Wang, L.; Li, S.F.; Zhang, L.; Zhang, L.W. Nitrite Scavenging and Inhibition of N-Nitrosamines Formation by Phenolic Extracts From Diospyros lotus L. Leaves and Active Ingredients. Nat. Prod. Commun. 2020, 15, 1934578X20961186. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products—Invited review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.H.; Yeh, P.J.; Wang, C.H.; Chen, H.C.; Chen, S.F. Analysis of heterocyclic amines in meat products by liquid chromatography—Tandem mass spectrometry. J. Food Drug Anal. 2019, 27, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Kong, B.; Chen, Q.; Han, Q.; Diao, X. N-nitrosoamine inhibition and quality preservation of Harbin dry sausages by inoculated with Lactobacillus pentosus, Lactobacillus curvatus and Lactobacillus sake. Food Control 2017, 73, 1514–1521. [Google Scholar] [CrossRef]
- Fan, T.; Sun, G.; Zhao, L.; Cui, X.; Zhong, R. Qsar and classification study on prediction of acute oral toxicity of n-nitroso compounds. Int. J. Mol. Sci. 2018, 19, 3015. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Lu, Q.; Gu, Y.; Pan, E.; Sun, Z.; Zhang, H.; Zhou, J.; Du, Y.; Zhang, Y.; Feng, Y.; et al. Distribution of N-nitrosamines in drinking water and human urinary excretions in high incidence area of esophageal cancer in Huai’an, China. Chemosphere 2019, 235, 288–296. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Wang, S. Effects of rosemary extract, grape seed extract and green tea polyphenol on the formation of N-nitrosamines and quality of western-style smoked sausage. J. Food Process. Preserv. 2020, 44, e14459. [Google Scholar] [CrossRef]
- Tang, R.; Peng, J.; Chen, L.; Liu, D.; Wang, W.; Guo, X. Combination of Flos Sophorae and chili pepper as a nitrite alternative improves the antioxidant, microbial communities and quality traits in Chinese sausages. Food Res. Int. 2021, 141, 110131. [Google Scholar] [CrossRef]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the microbial community composition in Italian Cinta Senese sausages dry-fermented with natural extracts as alternatives to sodium nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive compounds from vine shoots, grape stalks, and wine lees: Their potential use in agro-food chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef]
- Riazi, F.; Zeynali, F.; Hoseini, E.; Behmadi, H.; Savadkoohi, S. Oxidation phenomena and color properties of grape pomace on nitrite-reduced meat emulsion systems. Meat Sci. 2016, 121, 350–358. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Franco, D.; Bermúdez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of natural antioxidants in Spanish salchichón elaborated with encapsulated n-3 long chain fatty acids in konjac glucomannan matrix. Meat Sci. 2017, 124, 54–60. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Kubota, E.H.; da Silva, C.G.; dos Santos Alves, J.; Hautrive, T.P.; Rodrigues, G.S.; Campagnol, P.C.B. Banana inflorescences: A cheap raw material with great potential to be used as a natural antioxidant in meat products. Meat Sci. 2020, 161. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Difonzo, G.; Squeo, G.; Pasqualone, A.; Summo, C.; Paradiso, V.M.; Caponio, F. The challenge of exploiting polyphenols from olive leaves: Addition to foods to improve their shelf-life and nutritional value. J. Sci. Food Agric. 2021, 101, 3099–3116. [Google Scholar] [CrossRef]
- Caponio, F.; Difonzo, G.; Calasso, M.; Cosmai, L.; De Angelis, M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Food Res. Int. 2019, 116, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Testa, B.; Lombardi, S.J.; Macciola, E.; Succi, M.; Tremonte, P.; Iorizzo, M. Efficacy of olive leaf extract (Olea europaea L. cv Gentile di Larino) in marinated anchovies (Engraulis encrasicolus, L.) process. Heliyon 2019, 5, e01727. [Google Scholar] [CrossRef] [Green Version]
- Khemakhem, I.; Fuentes, A.; Lerma-García, M.J.; Ayadi, M.A.; Bouaziz, M.; Barat, J.M. Olive leaf extracts for shelf life extension of salmon burgers. Food Sci. Technol. Int. 2019, 25, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Aouidi, F.; Okba, A.; Hamdi, M. Valorization of functional properties of extract and powder of olive leaves in raw and cooked minced beef meat. J. Sci. Food Agric. 2017, 97, 3195–3203. [Google Scholar] [CrossRef]
- Kurt, Ş.; Ceylan, H.G. Effects of olive leaf extract on the oxidation stability and microbiological quality of dry fermented sausage (Sucuck). Carpathian J. Food Sci. Technol. 2017, 9, 178–188. [Google Scholar]
- Conte, P.; Pulina, S.; Del Caro, A.; Fadda, C.; Urgeghe, P.P.; De Bruno, A.; Difonzo, G.; Caponio, F.; Romeo, R.; Piga, A. Gluten-Free Breadsticks Fortified with Phenolic-Rich Extracts. Foods 2021, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Centrone, M.; D’Agostino, M.; Difonzo, G.; De Bruno, A.; Di Mise, A.; Ranieri, M.; Montemurro, C.; Valenti, G.; Poiana, M.; Caponio, F.; et al. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods 2020, 10, 11. [Google Scholar] [CrossRef]
- Union List of Food Additives (Text with EEA Relevance); Commission Regulation (EU) No 1129/2011; European Commission: Brussels, Belgium, 11 November 2011.
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- ISO 15214; Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria. Colony-Count Technique at 30 °C. International Standards. Microbiology of food and animal feeding stuffs.; Copyright Office: Genève, Switzerland, 2008.
- ISO 15213; International Standard Institute Horizontal Method for the Enumeration of Sulfite-Reducing Bacteria Growing under Anaerobic Conditions. Microbiology of food and animal feeding stuffs; Copyright Office: Genève, Switzerland, 2003.
- ISO 4832; Horizontal Method for the Enumeration of Coliforms Colony Count Technique. Microbiology of food and animal feeding stuffs; Copyright Office: Genève, Switzerland, 2006.
- ISO 16649-2; Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli-Part 2: Colony-Count Technique at 44 °C Using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. Zagreb: Croatian St. Microbiology of food and animal feeding stuffs.; Copyright Office: Genève, Switzerland, 2001.
- ISO 6888-2; Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species)—Part 2: Method Using Rabbit Plasma Fibrinogen Agar Medium. Microbiol. food Chain.; Copyright Office: Genève, Switzerland, 2004.
- EN ISO 11290-1; Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 1: Detection Method. Microbiol. food Chain.; Copyright Office: Genève, Switzerland, 2017.
- Baldini, M.; Fabietti, F.; Giammarioli, S.; Onori, R.; Orefice, L.; Stacchini, A. Metodi di Analisi Utilizzati per il Controllo Chimico Degli Alimenti—Rapporti ISTISAN 96/34; Istituto Superiore di Sanità (ISS): Rome, Italy, 1996. [Google Scholar]
- EN 12014-4:2005; Foodstuffs—Determination of Nitrate and/or Nitrite Content—Part 4: Ion-Exchange Chromatographic (Ic) Method for the Determination of Nitrate and Nitrite Content of Meat Products; CEN-CENELEC Management Centre: Brussels, Belgium, 2005.
- Jin, S.; Choi, J.S.; Yang, H.; Park, T.; Yim, D. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Penna, A.L.B.; da Silva Barretto, A.C. Applicability of potentially probiotic Lactobacillus casei in low-fat Italian type salami with added fructooligosaccharides: In vitro screening and technological evaluation. Meat Sci. 2020, 168, 108186. [Google Scholar] [CrossRef]
- Folch, J.; Less, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS Press Champaign: Champaign, IL, USA, 1993. [Google Scholar]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.; Buruleanu, L.; Rustad, T.; Manea, I.; Barascu, E. Overview on the microbiological quality of some meat products with impact on the food safety and health of people. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017; pp. 105–108. [Google Scholar]
- Aminzare, M.; Tajik, H.; Aliakbarlu, J.; Hashemi, M.; Raeisi, M. Effect of cinnamon essential oil and grape seed extract as functional-natural additives in the production of cooked sausage-impact on microbiological, physicochemical, lipid oxidation and sensory aspects, and fate of inoculated Clostridium perfringens. J. Food Saf. 2018, 38, e12459. [Google Scholar] [CrossRef]
- Microbiological Criteria for Foodstuffs; Commission Regulation (EU) No 1441/2007 of 5 December 2005; European Commission: Brussels, Belgium, 5 December 2007.
- Pateiro, M.; Bermú, R.; Lorenzo, J.M.; Franco, D. Effect of Addition of Natural Antioxidants on the Shelf-Life of “Chorizo”, a Spanish Dry-Cured Sausage. Antioxidants 2015, 42–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, M.M.; Munekata, P.E.S.; Jacinto-Valderrama, R.A.; Efraim, P.; Pateiro, M.; Lorenzo, J.M.; Pollonio, M.A.R. Beetroot and radish powders as natural nitrite source for fermented dry sausages. Meat Sci. 2021, 171, 108275. [Google Scholar] [CrossRef]
- Ozaki, M.M.; dos Santos, M.; Ribeiro, W.O.; de Azambuja Ferreira, N.C.; Picone, C.S.F.; Domínguez, R.; Lorenzo, J.M.; Pollonio, M.A.R. Radish powder and oregano essential oil as nitrite substitutes in fermented cooked sausages. Food Res. Int. 2021, 140, 109855. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.M.; Munekata, P.E.S.; Lopes, A.d.S.; do Nascimento, M.d.S.; Pateiro, M.A.R. Using chitosan and radish powder to improve stability of fermented cooked sausages. Meat Sci. 2020, 167, 108165. [Google Scholar] [CrossRef]
- Difonzo, G.; Squeo, G.; Calasso, M.; Pasqualone, A.; Caponio, F. Physico-chemical, microbiological and sensory evaluation of ready-to-use vegetable pâté added with olive leaf extract. Foods 2019, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Natrella, G.; Difonzo, G.; Calasso, M.; Costantino, G.; Caponio, F.; Faccia, M. Evolution of VOC and sensory characteristics of stracciatella cheese as affected by different preservatives. Foods 2020, 9, 1446. [Google Scholar] [CrossRef]
- Borjan, D.; Leitgeb, M.; Knez, Ž. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Liu, Y.; Mckeever, L.C.; Malik, N.S.A. Assessment of the Antimicrobial Activity of Olive Leaf Extract Against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, J.; Žauhar, G.; Žuvić, M. Optimization of ultrasonic-assisted extraction of major phenolic compounds from olive leaves (Olea europaea L.) using response surface methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, Y.; Jia, Q.; Lamacchia, V. A systems biology approach to investigate the antimicrobial activity of oleuropein. J. Ind. Microbiol. Biotechnol. 2016, 43, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Substitution of synthetic nitrates and antioxidants by spices, fruits and vegetables in Clean label Spanish chorizo. Food Res. Int. 2021, 139, 109835. [Google Scholar] [CrossRef] [PubMed]
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef]
- Hwang, K.E.; Kim, T.K.; Kim, H.W.; Seo, D.H.; Kim, Y.B.; Jeon, K.H.; Choi, Y.S. Effect of natural pre-converted nitrite sources on color development in raw and cooked pork sausage. Asian-Australasian J. Anim. Sci. 2018, 31, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- EFSA Opinion of the Scientific Panel on Biological Hazards on the request from the Commission related to the effects of Nitrites/Nitrates on the Microbiological Safety of Meat Products. EFSA J. 2003, 1–31.
- Riazi, F.; Zeynali, F.; Hoseini, E.; Behmadi, H. Effect of Dry Red Grape Pomace as a Nitrite Substitute on the Microbiological and Physicochemical Properties and Residual Nitrite of Dry-cured Sausage. Nutr. Food Sci. Res. 2016, 3, 37–44. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pateiro, M.; Campagnol, P.C.B.; Barba, F.J.; Tomasevic, I.; Montesano, D.; Lucini, L.; Lorenzo, J.M. Effect of partial replacement of meat by carrot on physicochemical properties and fatty acid profile of fresh turkey sausages: A chemometric approach. J. Sci. Food Agric. 2020, 100, 4968–4977. [Google Scholar] [CrossRef]
- Deng, S.; Bai, X.; Li, Y.; Wang, B.; Kong, B.; Liu, Q.; Xia, X. Changes in moisture, colour, residual nitrites and N-nitrosamine accumulation of bacon induced by nitrite levels and dry-frying temperatures. Meat Sci. 2021, 181, 108604. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Difonzo, G.; Neri, L.; Faieta, M.; Caponio, F.; Pittia, P. From by-product to food ingredient: Evaluation of compositional and technological properties of olive-leaf phenolic extracts. J. Sci. Food Agric. 2019, 99, 6620–6627. [Google Scholar] [CrossRef] [PubMed]
- Martínez Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Innovative Natural Functional Ingredients from Olive and Citrus Extracts in Spanish-Type Dry-Cured Sausage “Fuet". Antioxidants 2021, 10, 180. [Google Scholar] [CrossRef]
- Kunrath, C.A.; Savoldi, D.C.; Paulo, J.; Mileski, F.; Novello, C.R.; Alfaro, T.; Marchi, J.F.; Tonial, I.B. Application and evaluation of propolis, the natural antioxidant in Italian-type salami. Brazilian J. Food Technol. 2017, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hospital, X.F.; Carballo, J.; Fernández, M.; Arnau, J.; Gratacós, M.; Hierro, E. Technological implications of reducing nitrate and nitrite levels in dry-fermented sausages: Typical microbiota, residual nitrate and nitrite and volatile profile. Food Control 2015, 57, 275–281. [Google Scholar] [CrossRef]
- Djeri, N.; Williams, S.K. Celery Juice Powder Used as Nitrite Substitute in Sliced Vacuum-Packaged Turkey Bologna Stored at 4C for 10 Weeks Under Retail Display Light. J. Food Qual. 2014, 37, 361–370. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Gull, B.; Pateiro, M.; Tomasevic, I.; Dom, R.; Lorenzo, J.M. Natural Antioxidants from Seeds and Their Application in Meat Products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef]
- Amaro-Blanco, G.; Delgado-Adámez, J.; Martín, M.J.; Ramírez, R. Active packaging using an olive leaf extract and high pressure processing for the preservation of sliced dry-cured shoulders from Iberian pigs. Innov. Food Sci. Emerg. Technol. 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Rocchetti, G.; Falasconi, I.; Dallolio, G.; Lucini, L.; Rebecchi, A. Impact of hurdle technologies and low temperatures during ripening on the production of nitrate-free pork salami: A microbiological and metabolomic comparison. LWT Food Sci. Technol. 2021, 141, 110939. [Google Scholar] [CrossRef]
- Garcia-Esteban, M.; Ansorena, D.; Astiasarán, I. Comparison of modified atmosphere packaging and vacuum packaging for long period storage of dry-cured ham: Effects on colour, texture and microbiological quality. Meat Sci. 2004, 67, 57–63. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, J.; Zhou, H.; Lu, A.; Xu, B. A comprehensive insight into the effects of microbial spoilage, myoglobin autoxidation, lipid oxidation, and protein oxidation on the discoloration of rabbit meat during retail display. Meat Sci. 2021, 172, 108359. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, W.; Tatol, M. Color difference Delta E. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Tsoukalas, D.S.; Katsanidis, E.; Marantidou, S.; Bloukas, J.G. Effect of freeze-dried leek powder (FDLP) and nitrite level on processing and quality characteristics of fermented sausages. Meat Sci. 2011, 87, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ali, M.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef]
- Tejerina, D.; García-torres, S.; Vaca, M.C.D.; Vázquez, F.M.; Cava, R. Effect of production system on physical-chemical, antioxidant and fatty acids composition of Longissimus dorsi and Serratus ventralis muscles from Iberian pig. Food Chem. 2012, 133, 293–299. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; Cava, R. Extensively reared Iberian pigs versus intensively reared white pigs for the manufacture of frankfurters. Meat Sci. 2006, 72, 356–364. [Google Scholar] [CrossRef]
- Asif, M. Chemical characteristics and nutritional potentials of unsaturated fatty acids. Chem. Int. Introd. 2015, 1, 118–133. [Google Scholar]
- Zubillaga, M.P.; Maerker, G.; Foglia, T.A.; Zubillaga, M.P.; Maerker, G.; Foglia, T.A. Antioxidant activity of sodium nitrite in meat. J. Am. Oil Chem. Soc. 1984, 61, 772–776. [Google Scholar] [CrossRef]


| OLE (mg/kg) | NO (mg/kg) | |
|---|---|---|
| CTRL | 0 | 150 NO2/150 NO3 |
| Tr1 | 200 | 75 NO2/75 NO3 |
| Tr2 | 400 | 75 NO2/75 NO3 |
| Tr3 | 800 | 75 NO2/75 NO3 |
| Tr4 | 200 | 0 |
| Tr5 | 400 | 0 |
| Tr6 | 800 | 0 |
| Parameter | Amount |
|---|---|
| TPC (mg GAE/g) | 126.35 ± 1.39 |
| ABTS (µmol TE/g) | 709.17 ± 8.57 |
| DPPH (µmol TE/g) | 676.59 ± 3.25 |
| Oleuropein (mg/g) | 93.12 ± 2.21 |
| OLE (mg/kg) | NO (mg/kg) | Residual Nitrate (mg/kg) | Residual Nitrite (mg/kg) | |
|---|---|---|---|---|
| CTRL | 0 | 300 | 15 | 20 |
| Tr1 | 200 | 150 | 6 | <10 |
| Tr2 | 400 | 150 | 10 | <10 |
| Tr3 | 800 | 150 | 9 | 11 |
| Tr4 | 200 | 0 | <5 | <10 |
| Tr5 | 400 | 0 | <5 | <10 |
| Tr6 | 800 | 0 | <5 | <10 |
| CTRL | Tr1 | Tr2 | Tr3 | Tr4 | Tr5 | Tr6 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Moisture (%) | 22.37 ± 0.18 BC | 20.25 ± 0.91 C | 22.08 ± 0.76 BC | 21.75 ± 0.82 BC | 23.67 ± 1.08 AB | 25.27 ± 0.90 A | 20.54 ± 0.31 C | OLE = 0.025 NO = 0.019 |
| Weight loss (%) | 44.81 ± 0.63 A | 45.37 ± 0.21 A | 44.70 ± 1.37 A | 45.60 ± 0.55 A | 39.47 ± 0.63 B | 38.47 ± 1.87 B | 41.19 ± 0.94 B | OLE = 0.041 NO < 0.001 |
| pH | 5.24 ± 0.00 C | 5.12 ± 0.00 D | 5.18 ± 0.03 C | 5.20 ± 0.05 C | 5.30 ± 0.01 B | 5.43 ± 0.00 A | 5.22 ± 0.00 C | OLE = 0.021 NO < 0.001 |
| aw | 0.75 ± 0.00 B | 0.75 ± 0.00 B | 0.75 ± 0.00 B | 0.75 ± 0.00 B | 0.81 ± 0.00 A | 0.81 ± 0.00 A | 0.75 ± 0.00 B | OLE = 0.013 NO < 0.001 |
| CTRL | Tr1 | Tr2 | Tr3 | Tr4 | Tr5 | Tr6 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Lightness (L*) | 32.58 ± 0.02 B | 35.37 ± 2.92 AB | 33.98 ± 0.39 B | 33.25 ± 0.74 B | 35.18 ± 0.62 AB | 37.92 ± 0.58 A | 35.43 ± 0.75 AB | OLE = 0.213 NO < 0.001 |
| Redness (a*) | 8.27 ± 0.60 A | 8.48 ± 1.58 A | 8.10 ± 1.72 A | 6.55 ± 1.27 AB | 4.24 ± 0.19 B | 3.35 ± 1.24 B | 4.42 ± 1.06 B | OLE = 0.928 NO < 0.001 |
| Yellowness (b*) | 7.55 ± 0.18 AB | 9.27 ± 1.33 AB | 6.85 ± 0.30 B | 6.57 ± 0.30 B | 8.26 ± 0.74 AB | 9.90 ± 2.13 A | 6.92 ± 0.03 B | OLE < 0.001 NO = 0.025 |
| ΔE vs. CTRL | 3.63 ± 2.97 | 2.30 ± 0.60 | 2.23 ± 0.51 | 4.94 ± 0.55 | 7.73 ± 2.41 | 4.89 ± 0.06 |
| CTRL | Tr1 | Tr2 | Tr3 | Tr4 | Tr5 | Tr6 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Hardness (N) | 108.00 ± 0.00 A | 74.13 ± 12.83 BC | 84.00 ± 13.44 AB | 97.18 ± 5.45 AB | 43.87 ± 1.95 C | 69.78 ± 22.42 BC | 71.36 ± 3.01 BC | OLE = 0.007 NO < 0.001 |
| Springiness | 0.37 ± 0.04 C | 0.43 ± 0.02 AB | 0.42 ± 0.02 ABC | 0.47 ± 0.00 A | 0.39 ± 0.03 BC | 0.37 ± 0.01 BC | 0.46 ± 0.00 A | OLE < 0.001 NO < 0.001 |
| Gumminess | 18.64 ± 2.48 B | 16.05 ± 3.89 BC | 17.62 ± 2.35 B | 29.68 ± 1.38 A | 10.84 ± 0.23 C | 15.68 ± 3.02 BC | 20.95 ± 0.39 B | OLE < 0.001 NO < 0.001 |
| Chewiness (N) | 7.07 ± 1.65 BCD | 7.13 ± 2.11 BCD | 7.61 ± 0.70 BC | 14.69 ± 0.41 A | 4.28 ± 0.41 D | 5.94 ± 1.11 CD | 9.75 ± 0.15 B | OLE < 0.001 NO < 0.001 |
| Cohesivity (N) | 0.17 ± 0.03 D | 0.21 ± 0.01 CD | 0.20 ± 0.01 CD | 0.29 ± 0.03 A | 0.25 ± 0.01 ABC | 0.23 ± 0.03 BC | 0.28 ± 0.01 AB | OLE < 0.001 NO = 0.135 |
| CTRL | Tr1 | Tr2 | Tr3 | Tr4 | Tr5 | Tr6 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Myristic C14:0 | 1.34 ± 0.01 AB | 1.41 ± 0.05 A | 1.33 ± 0.02 AB | 1.33 ± 0.05 AB | 1.32 ± 0.02 B | 1.34 ± 0.01 AB | 1.34 ± 0.01 AB | OLE = 0.305 NO = 0.178 |
| Palmitic C16:0 | 23.32 ± 0.03 B | 24.41 ± 0.64 A | 23.79 ± 0.17 AB | 23.65 ± 0.51 AB | 23.36 ± 0.23 B | 23.83 ± 0.08 AB | 23.55 ± 0.08 AB | OLE = 0.513 NO = 0.085 |
| Stearic C18:0 | 10.80 ± 0.18 B | 11.76 ± 0.10 A | 11.50 ± 0.09 A | 11.55 ± 0.03 A | 11.19 ± 0.55 AB | 11.73 ± 0.07 A | 11.22 ± 0.06 AB | OLE = 0.420 NO = 0.122 |
| Oleic C18:1 | 48.44 ± 0.22 A | 47.54 ± 0.13 AB | 48.48 ± 0.19 A | 48.20 ± 0.39 A | 47.74 ± 0.55 A | 46.77 ± 0.15 B | 47.82 ± 0.47 A | OLE = 0.425 NO = 0.033 |
| Linoleic C18:2 | 9.95 ± 0.08 A | 9.04 ± 0.73 A | 9.07 ± 0.09 A | 9.21 ± 0.99 A | 10.27 ± 0.15 A | 10.25 ± 0.12 A | 10.03 ± 0.44 A | OLE = 0.787 NO < 0.001 |
| Linolenic C18:3 | 1.10 ± 0.01 AB | 1.07 ± 0.02 B | 1.08 ± 0.02 B | 1.12 ± 0.00 A | 1.07 ± 0.01 B | 1.02 ± 0.01 C | 1.07 ± 0.02 B | OLE = 0.008 NO = 0.002 |
| ƩSFA | 35.93 ± 0.13 B | 38.08 ± 0.86 A | 37.07 ± 0.30 AB | 37.12 ± 0.68 AB | 36.32 ± 0.76 B | 37.35 ± 0.02 AB | 36.56 ± 0.01 B | OLE = 0.595 NO = 0.059 |
| ƩMUFA | 52.03 ± 0.25 A | 50.95 ± 0.09 BC | 51.93 ± 0.20 AB | 51.62 ± 0.40 AB | 51.33 ± 0.60 ABC | 50.37 ± 0.14 C | 51.39 ± 0.48 AB | OLE = 0.467 NO = 0.103 |
| ƩPUFA | 12.02 ± 0.12 A | 10.96 ± 0.77 A | 10.99 ± 0.09 A | 11.24 ± 1.08 A | 12.34 ± 0.16 A | 12.27 ± 0.12 A | 12.04 ± 0.48 A | OLE = 0.719 NO < 0.001 |
| n-3 PUFA | 1.19 ± 0.02 AB | 1.14 ± 0.01 CD | 1.1 ± 0.01 CD | 1.20 ± 0.02 A | 1.18 ± 0.01 ABC | 1.13 ± 0.00 D | 1.16 ± 0.01 BCD | OLE = 0.032 NO = 0.393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Difonzo, G.; Totaro, M.P.; Caponio, F.; Pasqualone, A.; Summo, C. Olive Leaf Extract (OLE) Addition as Tool to Reduce Nitrate and Nitrite in Ripened Sausages. Foods 2022, 11, 451. https://doi.org/10.3390/foods11030451
Difonzo G, Totaro MP, Caponio F, Pasqualone A, Summo C. Olive Leaf Extract (OLE) Addition as Tool to Reduce Nitrate and Nitrite in Ripened Sausages. Foods. 2022; 11(3):451. https://doi.org/10.3390/foods11030451
Chicago/Turabian StyleDifonzo, Graziana, Michela Pia Totaro, Francesco Caponio, Antonella Pasqualone, and Carmine Summo. 2022. "Olive Leaf Extract (OLE) Addition as Tool to Reduce Nitrate and Nitrite in Ripened Sausages" Foods 11, no. 3: 451. https://doi.org/10.3390/foods11030451
APA StyleDifonzo, G., Totaro, M. P., Caponio, F., Pasqualone, A., & Summo, C. (2022). Olive Leaf Extract (OLE) Addition as Tool to Reduce Nitrate and Nitrite in Ripened Sausages. Foods, 11(3), 451. https://doi.org/10.3390/foods11030451