Bread Sourdough Lactic Acid Bacteria—Technological, Antimicrobial, Toxin-Degrading, Immune System-, and Faecal Microbiota-Modelling Biological Agents for the Preparation of Food, Nutraceuticals and Feed
Abstract
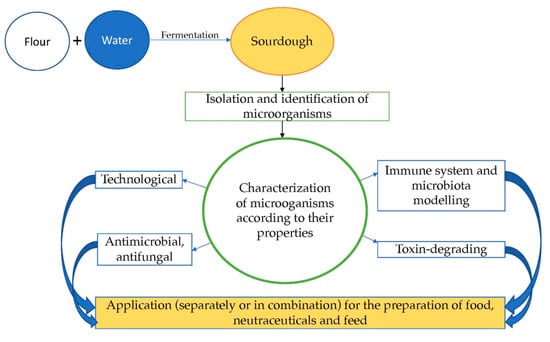
:1. Introduction
2. Sourdough LAB—Technological Starters for Food Preparation
2.1. Pure Sourdough Starters and Their Combinations for Preparation of Higher-Value Safer Bread
2.2. Challenges Associated with Lacto-Fermentation of Meat and Meat Products by Using Sourdough Lactic Acid Bacteria
2.2.1. Sourdough LAB for Reducing the Concentration of PAHs and BAs in Meat Products
2.2.2. Sourdough LAB-Based Marinades for Meat Pre-Treatment
2.2.3. Sourdough LAB–Plant-Based Bioproducts for Increasing the Functional Value of Meat Products
2.3. Application of Sourdough LAB in the Preparation of Dairy Products
2.4. Application of Sourdough LAB for the Valorisation of by-Products, including Toxin-Degradation Properties
2.5. Application of Sourdough LAB for Higher-Value Food and Nutraceutical Formulations
3. Sourdough LAB and Their Prospective Antimicrobial Combinations with Plant- and Animal-Based Ingredients
4. Sourdough LAB as Biological Agents for Modelling the Immune System and Digestion Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Vuyst, L.; Schrijvers, V.; Paramithiotis, S.; Hoste, B.; Vancanneyt, M.; Swings, J.; Kalantzopoulos, G.; Tsakalidou, E.; Messens, W. The biodiversity of lactic acid bacteria in Greek traditional wheat sourdoughs is reflected in both composition and metabolite formation. Appl. Environ. Microbiol. 2002, 68, 6059–6069. [Google Scholar] [CrossRef] [Green Version]
- Edema, M.O. A modified sourdough procedure for non-wheat bread from maize meal. Food Bioprocess Technol. 2011, 4, 1264–1272. [Google Scholar] [CrossRef]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L.; Van Sinderen, D. Characterization of bacteriocin-like inhibitory substances (BLIS) from sourdough lactic acid bacteria and evaluation of their in vitro and in situ activity. J. Appl. Microbiol. 2004, 96, 521–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Holzapfel, W.H.; Haberer, P.; Snel, J.; Schillinger, U.; Huis in’t Veld, J.H.J. Overview of gut flora and probiotics. Int. J. Food Microbiol. 1998, 41, 85–101. [Google Scholar] [CrossRef]
- Lee, C.-H. Lactic acid fermented foods and their benefits in Asia. Food Control 1997, 8, 259–269. [Google Scholar] [CrossRef]
- Oyewole, O.B. Lactic fermented foods in Africa and their benefits. Food Control 1997, 8, 289–297. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Classification of fermented foods: Worldwide review of household fermentation techniques. Food Control 1997, 8, 311–317. [Google Scholar] [CrossRef]
- Vogel, R.F.; Müller, M.; Stolz, P.; Ehrmann, M. Ecology in sourdoughs produced by traditional and modern technologies. Adv. Food Sci. 1996, 18, 152–159. [Google Scholar]
- Akinola, S.; Osundahunsi, O. Lactic acid bacteria and yeast diversities in spontaneously fermented millet sourdough. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1030–1035. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.; Di Cagno, R.; Gallo, G.; Curci, M.; Siragusa, S.; Crecchio, C.; Parente, E.; Gobbetti, M. Molecular and functional characterization of Lactobacillus sanfranciscensis strains isolated from sourdoughs. Int. J. Food Microbiol. 2007, 114, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the antimicrobial activity of lactic acid bacteria in combination with berries/fruits and dairy industry by-products. J. Sci. Food Agric. 2019, 99, 3992–4002. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Varinauskaite, I.; Pileckaite, G.; Paskeviciute, L.; Rutkauskaite, G.; Kanaporis, T.; et al. Plants and lactic acid bacteria combination for new antimicrobial and antioxidant properties product development in a sustainable manner. Foods 2020, 9, 433. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P.; et al. Development of antimicrobial gummy candies with addition of bovine colostrum, essential oils and probiotics. Int. J. Food Sci. Technol. 2018, 53, 1227–1235. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Zommiti, M.; Ferchichi, M.; Sebei, K.; Feuilloley, M.G.J.; Connil, N.; Boukerb, A.M. Draft Genome sequences of five potentially probiotic enterococcus faecium strains isolated from an artisanal Tunisian meat (dried ossban). Microbiol. Resour. Announc. 2020, 9, e01348-19. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Vizbickiene, D.; Bartkevics, V.; Pugajeva, I.; Krungleviciute, V.; Zadeike, D.; Zavistanaviciute, P.; Juodeikiene, G. Application of pediococcus acidilactici LUHS29 immobilized in apple pomace matrix for high value wheat-barley sourdough bread. LWT-Food Sci. Technol. 2017, 83, 157–164. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Pugajeva, I.; Zadeike, D.; Juodeikiene, G. Lactic acid bacteria combinations for wheat sourdough preparation and their influence on wheat bread quality and acrylamide formation. J. Food Sci. 2017, 82, 2371–2378. [Google Scholar] [CrossRef]
- Nionelli, L.; Wang, Y.; Pontonio, E.; Immonen, M.; Rizzello, C.G.; Maina, H.N.; Katina, K.; Coda, R. Antifungal effect of bioprocessed surplus bread as ingredient for bread-making: Identification of active compounds and impact on shelf-life. Food Control 2020, 118, 107437. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Zadeike, D.; Juodeikiene, G. Application of antifungal lactobacilli in combination with coatings based on apple processing by-products as a bio-preservative in wheat bread production. J. Food Sci. Technol. 2019, 56, 2989–3000. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Mickiene, R.; Zadeike, D.; Juodeikiene, G. A concept of mould spoilage prevention and acrylamide reduction in wheat bread: Application of lactobacilli in combination with a cranberry coating. Food Control 2018, 91, 284–293. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Pugajeva, I.; Zadeike, D.; Juodeikiene, G.; Cizeikiene, D. The influence of scalded flour, fermentation, and plants belonging to Lamiaceae family on the wheat bread quality and acrylamide content. J. Food Sci. 2018, 83, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Bartkevics, V.; Pugajeva, I.; Borisova, A.; Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Zadeike, D.; et al. The quality of wheat bread with ultrasonicated and fermented by-products from plant drinks production. Front. Microbiol. 2021, 12, 586. [Google Scholar] [CrossRef]
- Katayama, M.; Wilson, L.A. Utilization of okara, a byproduct from soymilk production, through the development of soy-based snack food. J. Food Sci. 2008, 73, S152–S157. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Trakselyte-Rupsiene, K.; Navickaite, B.; Zadeike, D.; Bendoraitiene, J.; Bartkiene, E.; Lele, V.; Rueller, L.; Robert, J.; Arnoldi, A.; et al. Functionalization of soya press cake (okara) by ultrasonication for enhancement of submerged fermentation with Lactobacillus paracasei LUHS244 for wheat bread production. LWT 2021, 152, 112337. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Mozuriene, E.; Lele, V.; Zadeike, D.; Juodeikiene, G. The safety, technological, nutritional, and sensory challenges associated with lacto-fermentation of meat and meat products by using pure lactic acid bacteria strains and plant-lactic acid bacteria bioproducts. Front. Microbiol. 2019, 10, 1036. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria: Their applications in foods. J. Bacteriol. Mycol. Open Access 2018, 6, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Bartkevics, V.; Mozuriene, E.; Krungleviciute, V.; Novoslavskij, A.; Santini, A.; Rozentale, I.; Juodeikiene, G.; Cizeikiene, D. The impact of lactic acid bacteria with antimicrobial properties on biodegradation of polycyclic aromatic hydrocarbons and biogenic amines in cold smoked pork sausages. Food Control 2017, 71, 285–292. [Google Scholar] [CrossRef]
- Chiocchetti, G.M.; Jadán-Piedra, C.; Monedero, V.; Zúñiga, M.; Vélez, D.; Devesa, V. Use of lactic acid bacteria and yeasts to reduce exposure to chemical food contaminants and toxicity. Crit. Rev. Food Sci. Nutr. 2019, 59, 1534–1545. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef] [Green Version]
- Özogul, F.; Hamed, I. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, K.A.; Reistad, R.; Risøen, U.; Rønning, H.F. Dietary polyamines. Food Chem. 2002, 78, 273–280. [Google Scholar] [CrossRef]
- Kafouris, D.; Koukkidou, A.; Christou, E.; Hadjigeorgiou, M.; Yiannopoulos, S. Determination of polycyclic aromatic hydrocarbons in traditionally smoked meat products and charcoal grilled meat in Cyprus. Meat Sci. 2020, 164, 108088. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef] [PubMed]
- De Mey, E.; De Maere, H.; Paelinck, H.; Fraeye, I. Volatile N-nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2909–2923. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, P.; Zhou, Y.; Ma, F.; Chen, C. Effect of inoculating Lactobacillus pentosus R3 on N-nitrosamines and bacterial communities in dry fermented sausages. Food Control 2018, 87, 126–134. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Veyrand, B.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.K.J.; Dembélé, Y.K.; Eyangoh, S.; Verger, P.; et al. Polycyclic aromatic hydrocarbons in foods from the first regional total diet study in Sub-Saharan Africa: Contamination profile and occurrence data. Food Control 2019, 103, 133–144. [Google Scholar] [CrossRef]
- Lu, S.; Wu, D.; Li, G.; Lv, Z.; Gong, P.; Xia, L.; Sun, Z.; Chen, G.; Chen, X.; You, J.; et al. Facile and sensitive determination of N-nitrosamines in food samples by high-performance liquid chromatography via combining fluorescent labeling with dispersive liquid-liquid microextraction. Food Chem. 2017, 234, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, L.; Daguer, H.; Motta, G.E.; Merlo, T.C.; Lindner, J.D.D. Interactions of preservatives in meat processing: Formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res. Int. 2019, 125, 108608. [Google Scholar] [CrossRef]
- Choi, S.Y.; Chung, M.J.; Lee, S.-J.; Shin, J.H.; Sung, N.J. N-Nitrosamine inhibition by strawberry, garlic, kale, and the effects of nitrite-scavenging and N-nitrosamine formation by functional compounds in strawberry and garlic. Food Control 2007, 18, 485–491. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi Asli, M.; Hosseini, H.; Shadnoush, M.; Mortazavian, A.M. Potential anticarcinogenic effects of lactic acid bacteria and probiotics in detoxification of process-induced food toxicants. Iran. J. Cancer Prev. 2016, 9, e7920. [Google Scholar] [CrossRef]
- ur Rahman, U.; Sahar, A.; Khan, M.I.; Nadeem, M. Production of heterocyclic aromatic amines in meat: Chemistry, health risks and inhibition. A review. LWT-Food Sci. Technol. 2014, 59, 229–233. [Google Scholar] [CrossRef]
- Šimko, P. Determination of polycyclic aromatic hydrocarbons in smoked meat products and smoke flavouring food additives. J. Chromatogr. B 2002, 770, 3–18. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Walczak, J.; Buszewski, B. Microbiology neutralization of zearalenone using Lactococcus lactis and Bifidobacterium sp. Anal. Bioanal. Chem. 2018, 410, 943–952. [Google Scholar] [CrossRef]
- Lo, P.-R.; Yu, R.-C.; Chou, C.-C.; Huang, E.-C. Determinations of the antimutagenic activities of several probiotic bifidobacteria under acidic and bile conditions against benzo[a]pyrene by a modified Ames test. Int. J. Food Microbiol. 2004, 93, 249–257. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, J.; Pan, X.; Pei, J.; Zhang, B. Binding of benzo(a)pyrene by lactobacilli strains. Wei Sheng Wu Xue Bao 2011, 51, 956–964. [Google Scholar]
- Zhao, H.; Zhou, F.; Qi, Y.; Dziugan, P.; Bai, F.; Walczak, P.; Zhang, B. Screening of Lactobacillus strains for their ability to bind benzo(a)pyrene and the mechanism of the process. Food Chem. Toxicol. 2013, 59, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Dapkevicius, M.L.N.E.; Nout, M.J.R.; Rombouts, F.M.; Houben, J.H.; Wymenga, W. Biogenic amine formation and degradation by potential fish silage starter microorganisms. Int. J. Food Microbiol. 2000, 57, 107–114. [Google Scholar] [CrossRef]
- García-Ruiz, A.; González-Rompinelli, E.M.; Bartolomé, B.; Moreno-Arribas, M.V. Potential of wine-associated lactic acid bacteria to degrade biogenic amines. Int. J. Food Microbiol. 2011, 148, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalač, P.; Špička, J.; Křížek, M.; Pelikánová, T. The effects of lactic acid bacteria inoculants on biogenic amines formation in sauerkraut. Food Chem. 2000, 70, 355–359. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, S.H.; Kang, K.H.; Lee, S.; Kim, S.J.; Kim, J.G.; Chung, M.J. Kimchi probiotic bacteria contribute to reduced amounts of N-nitrosodimethylamine in lactic acid bacteria-fortified kimchi. LWT 2017, 84, 196–203. [Google Scholar] [CrossRef]
- Liao, E.; Xu, Y.; Jiang, Q.; Xia, W. Effects of inoculating autochthonous starter cultures on N-nitrosodimethylamine and its precursors formation during fermentation of Chinese traditional fermented fish. Food Chem. 2019, 271, 174–181. [Google Scholar] [CrossRef]
- Lu, S.; Ji, H.; Wang, Q.; Li, B.; Li, K.; Xu, C.; Jiang, C. The effects of starter cultures and plant extracts on the biogenic amine accumulation in traditional Chinese smoked horsemeat sausages. Food Control 2015, 50, 869–875. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Q.; Li, F.; Zheng, D.; Kong, B. Biogenic amine inhibition and quality protection of Harbin dry sausages by inoculation with Staphylococcus xylosus and Lactobacillus plantarum. Food Control 2016, 68, 358–366. [Google Scholar] [CrossRef]
- Kuley, E.; Durmus, M.; Ucar, Y.; Kosker, A.R.; Aksun Tumerkan, E.T.; Regenstein, J.M.; Ozogul, F. Combined effects of plant and cell-free extracts of lactic acid bacteria on biogenic amines and bacterial load of fermented sardine stored at 3 ± 1 °C. Food Biosci. 2018, 24, 127–136. [Google Scholar] [CrossRef]
- Ozogul, F.; Tabanelli, G.; Toy, N.; Gardini, F. Impact of cell-free supernatant of lactic acid bacteria on putrescine and other polyamine formation by foodborne pathogens in ornithine decarboxylase broth. J. Agric. Food Chem. 2015, 63, 5828–5835. [Google Scholar] [CrossRef]
- Özogul, F.; Toy, N.; Özogul, Y.; Hamed, I. Function of cell-free supernatants of Leuconostoc, Lactococcus, Streptococcus, Pediococcus strains on histamine formation by foodborne pathogens in histidine decarboxylase broth. J. Food Process. Preserv. 2017, 41, e13208. [Google Scholar] [CrossRef]
- Toy, N.; Özogul, F.; Özogul, Y. The influence of the cell free solution of lactic acid bacteria on tyramine production by food borne-pathogens in tyrosine decarboxylase broth. Food Chem. 2015, 173, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, P.; Xie, Y.; Wang, X. Co-fermentation with Lactobacillus curvatus LAB26 and Pediococcus pentosaceus SWU73571 for improving quality and safety of sour meat. Meat Sci. 2020, 170, 108240. [Google Scholar] [CrossRef] [PubMed]
- Mozuriene, E.; Bartkiene, E.; Krungleviciute, V.; Zadeike, D.; Juodeikiene, G.; Damasius, J.; Baltusnikiene, A. Effect of natural marinade based on lactic acid bacteria on pork meat quality parameters and biogenic amine contents. LWT-Food Sci. Technol. 2016, 69, 319–326. [Google Scholar] [CrossRef]
- Bartkiene, E.; Gražina, J.; Zadeike, D.; Viskelis, P. The use of tomato powder fermented with Pediococcus pentosaceus and Lactobacillus sakei for the ready-to-cook minced meat product quality improvement. Food Technol. Biotechnol. 2015, 53. [Google Scholar] [CrossRef]
- Paseephol, T.; Small, D.; Sherkat, F. Process optimisation for fractionating Jerusalem artichoke fructans with ethanol using response surface methodology. Food Chem. 2007, 104, 73–80. [Google Scholar] [CrossRef]
- Stimbirys, A.; Bartkiene, E.; Siugzdaite, J.; Augeniene, D.; Vidmantiene, D.; Juodeikiene, G.; Maruska, A.; Stankevicius, M.; Cizeikiene, D. Safety and quality parameters of ready-to-cook minced pork meat products supplemented with Helianthus tuberosus L. tubers fermented by BLIS producing lactic acid bacteria. J. Food Sci. Technol. 2015, 52, 4306–4314. [Google Scholar] [CrossRef] [Green Version]
- Azimi, M.; Neyriz Naghadehi, M.; Moulodi, F.; Razavi Rohani, S.M.; Alizade Khaledabad, M. The effects of Satureja hortensis L. essential oil on the growth and survival of Salmonella typhimorium in minced poultry meat during refrigerated storage. J. Kermanshah Univ. Med. Sci. 2018, 22, e69640. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Bartkiene, E.; Mozuriene, E.; Juodeikiene, G.; Zadeike, D.; Maruska, A.; Stankevicius, M.; Ragazinskiene, O.; Cizeikiene, D. Pork meat products functional value and safety parameters improving by using lactic acid fermentation of savory plants. J. Food Sci. Technol. 2015, 52, 7143–7152. [Google Scholar] [CrossRef]
- Suleman, R.; Wang, Z.; Aadil, R.M.; Hui, T.; Hopkins, D.L.; Zhang, D. Effect of cooking on the nutritive quality, sensory properties and safety of lamb meat: Current challenges and future prospects. Meat Sci. 2020, 167, 108172. [Google Scholar] [CrossRef] [PubMed]
- Klupsaite, D.; Zavistanaviciute, P.; Sakiene, V.; Lele, V.; Mozuriene, E.; Klementaviciute, J.; Sidlauskiene, S.; Buckiuniene, V.; Tolpeznikaite, E.; Ruibys, R.; et al. Evaluation of the use of lactic acid bacteria and Thymus vulgaris essential oil on Suffolk and Ile de France lamb breed (Musculus gluteus) quality parameters. Int. J. Food Sci. Technol. 2020, 55, 3463–3474. [Google Scholar] [CrossRef]
- Bartkiene, E.; Laurikietyte, R.; Lele, V.; Zavistanaviciute, P.; Mozuriene, E.; Baltusnikiene, A. Agar-immobilized basil–lactic acid bacteria bioproducts as goat milk taste-masking agents and natural preservatives for the production of unripened goat cheese. J. Dairy Sci. 2018, 101, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Mozuriene, E.; Bartkiene, E.; Juodeikiene, G.; Zadeike, D.; Basinskiene, L.; Maruska, A.; Stankevicius, M.; Ragazinskiene, O.; Damasius, J.; Cizeikiene, D. The effect of savoury plants, fermented with lactic acid bacteria, on the microbiological contamination, quality, and acceptability of unripened curd cheese. LWT-Food Sci. Technol. 2016, 69, 161–168. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Pugajeva, I.; Borisova, A.; Zokaityte, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Klupsaite, D.; Zadeike, D.; et al. Challenges associated with byproducts valorization—comparison study of safety parameters of ultrasonicated and fermented plant-based byproducts. Foods 2020, 9, 614. [Google Scholar] [CrossRef]
- Bartkiene, E.; Mozuriene, E.; Lele, V.; Zokaityte, E.; Gruzauskas, R.; Jakobsone, I.; Juodeikiene, G.; Ruibys, R.; Bartkevics, V. Changes of bioactive compounds in barley industry by-products during submerged and solid state fermentation with antimicrobial Pediococcus acidilactici strain LUHS29. Food Sci. Nutr. 2020, 8, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Cernauskas, D.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; et al. Combination of extrusion and fermentation with Lactobacillus plantarum and L. uvarum strains for improving the safety characteristics of wheat bran. Toxins 2021, 13, 163. [Google Scholar] [CrossRef]
- Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Bartkevics, V.; Pugajeva, I.; Bērziņa, Z.; Gruzauskas, R.; Sidlauskiene, S.; et al. The influence of combined extrusion and fermentation processes on the chemical and biosafety parameters of wheat bran. LWT 2021, 146, 111498. [Google Scholar] [CrossRef]
- Bartkiene, E.; Juodeikiene, G.; Basinskiene, L.; Liukkonen, K.-H.; Adlercreutz, H.; Kluge, H. Enterolignans enterolactone and enterodiol formation from their precursors by the action of intestinal microflora and their relationship with non-starch polysaccharides in various berries and vegetables. LWT-Food Sci. Technol. 2011, 44, 48–53. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Klupsaite, D.; Bendoraitiene, J.; Navikaite-Snipaitiene, V.; Ruzauskas, M. Technology and characterisation of whole hemp seed beverages prepared from ultrasonicated and fermented whole seed paste. Int. J. Food Sci. Technol. 2020, 55, 406–419. [Google Scholar] [CrossRef]
- Gunasekaran, Y.K.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Zokaityte, E.; Klupsaite, D.; Bartkevics, V.; Guiné, R.P.F.; Bartkiene, E. Plant-based proteinaceous snacks: Effect of fermentation and ultrasonication on end-product characteristics. Food Sci. Nutr. 2020, 8, 4746–4756. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Sakiene, V.; Bartkevics, V.; Wiacek, C.; Rusko, J.; Lele, V.; Ruzauskas, M.; Juodeikiene, G.; Klupsaite, D.; Bernatoniene, J.; et al. Nutraceuticals in gummy candies form prepared from lacto-fermented lupine protein concentrates, as high-quality protein source, incorporated with Citrus paradise L. essential oil and xylitol. Int. J. Food Sci. Technol. 2018, 53, 2015–2025. [Google Scholar] [CrossRef]
- Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of milk permeate with selected lactic acid bacteria strains and apple by-products into beverages with antimicrobial properties and enriched with galactooligosaccharides. Microorganisms 2020, 8, 1182. [Google Scholar] [CrossRef] [PubMed]
- Zokaityte, E.; Siriakovaite, K.; Starkute, V.; Zavistanaviciute, P.; Lele, V.; Mozuriene, E.; Klupsaite, D.; Viskelis, P.; Ruibys, R.; Guiné, R.P.F.; et al. Characteristics of nutraceutical chewing candy formulations based on fermented milk permeate, psyllium husk, and apple by-products. Foods 2021, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Cernauskas, D.; Klupsaite, D.; Ruzauskas, M.; Alisauskaite, J.; Baltrusaitytė, A.; Dapsas, M.; et al. Antimicrobial, antioxidant, sensory properties, and emotions induced for the consumers of nutraceutical beverages developed from technological functionalised food industry by-products. Foods 2020, 9, 1620. [Google Scholar] [CrossRef]
- Grigas, J.; Ruzauskas, M.; Pautienius, A.; Bartkiene, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bernatoniene, J.; Ivanauskas, L.; et al. Investigation of immunomodulatory and gut microbiota-altering properties of multicomponent nutraceutical prepared from lactic acid bacteria, bovine colostrum, apple production by-products and essential oils. Foods 2021, 10, 1313. [Google Scholar] [CrossRef]
- Bartkiene, E.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; Zavistanaviciute, P.; Starkute, V.; Zokaityte, E.; Lele, V.; Dauksiene, A.; Grashorn, M.; et al. Study of the antibiotic residues in poultry meat in some of the EU countries and selection of the best compositions of lactic acid bacteria and essential oils against Salmonella enterica. Poult. Sci. 2020, 99, 4065–4076. [Google Scholar] [CrossRef]
- Tolpeznikaite, E.; Ruzauskas, M.; Pilkaityte, R.; Bartkevics, V.; Zavistanaviciute, P.; Starkute, V.; Lele, V.; Zokaityte, E.; Mozuriene, E.; Ruibys, R.; et al. Influence of fermentation on the characteristics of Baltic Sea macroalgae, including microbial profile and trace element content. Food Control 2021, 129, 108235. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Juodeikiene, G.; Zadeike, D.; Baliukoniene, V.; Bakutis, B.; Zelvyte, R.; Santini, A.; Cizeikiene, D. Application of hydrolases and probiotic Pediococcus acidilactici BaltBio01 strain for cereal by-products conversion to bioproduct for food/feed. Int. J. Food Sci. Nutr. 2018, 69, 165–175. [Google Scholar] [CrossRef]
- Lele, V.; Zelvyte, R.; Monkeviciene, I.; Kantautaite, J.; Stankevicius, R.; Ruzauskas, M.; Sederevicius, A.; Antanaitis, R.; Bartkiene, E. Milk production and ruminal parameters of dairy cows fed diets containing Lactobacillus sakei KTU05-6 and Pediococcus pentosaceus BaltBio02. Pol. J. Vet. Sci. 2019, 22, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ruzauskas, M.; Bartkiene, E.; Stankevicius, A.; Bernatoniene, J.; Zadeike, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Grigas, J.; Zokaityte, E.; et al. The influence of essential oils on gut microbial profiles in pigs. Animals 2020, 10, 1734. [Google Scholar] [CrossRef]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Badaras, S.; Klupsaite, D.; Mozuriene, E.; et al. Pigs’ feed fermentation model with antimicrobial lactic acid bacteria strains combination by changing extruded soya to biomodified local feed stock. Animals 2020, 10, 783. [Google Scholar] [CrossRef]
- Vadopalas, L.; Zokaityte, E.; Zavistanaviciute, P.; Gruzauskas, R.; Starkute, V.; Mockus, E.; Klementaviciute, J.; Ruzauskas, M.; Lele, V.; Cernauskas, D.; et al. Supplement based on fermented milk permeate for feeding newborn calves: Influence on blood, growth performance, and faecal parameters, including microbiota, volatile compounds, and fatty and organic acid profiles. Animals 2021, 11, 2544. [Google Scholar] [CrossRef]
- Zavistanaviciute, P.; Poskiene, I.; Kantautaite, J.; Antanaitis, R.; Bartkiene, E.; Lele, V. The influence of the newly isolated Lactobacillus plantarum LUHS135 and Lactobacillus paracasei LUHS244 strains on blood and faeces parameters in endurance horses. Pol. J. Vet. Sci. 2019, 22, 513–521. [Google Scholar] [PubMed]
- Zavistanaviciute, P.; Lele, V.; Antanaitis, R.; Televičius, M.; Ruzauskas, M.; Zebeli, Q.; Bartkiene, E. Separate and synergic effects of Lactobacillus uvarum LUHSS245 and arabinogalactan on the in vitro antimicrobial properties as well as on the fecal and metabolic profile of newborn calves. Animals 2020, 10, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Pugajeva, I.; Reinolds, I.; Badaras, S.; et al. Combination of antimicrobial starters for feed fermentation: Influence on piglet feces microbiota and health and growth performance, including mycotoxin biotransformation in vivo. Front. Vet. Sci. 2020, 7, 786. [Google Scholar] [CrossRef]
- Vadopalas, L.; Badaras, S.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Klupsaite, D.; Mozuriene, E.; et al. Influence of the fermented feed and vaccination and their interaction on parameters of Large White/Norwegian Landrace piglets. Animals 2020, 10, 1201. [Google Scholar] [CrossRef]



| Sourdough LAB Strains | Possible Applications | Reference |
|---|---|---|
| Pediococcus acidilactici LUHS29 | For barley sourdough fermentation and the preparation of higher-value bread | [19] |
| Combinations of LAB strains: Pediococcus pentosaceus LUHS183 and Leuconostoc mesenteroides LUHS242, P. pentosaceus LUHS183 and Lactobacillus brevis LUHS173, P. pentosaceus LUHS183 and Enterococcus pseudoavium LUHS234, P. pentosaceus LUHS183 and Lactobacillus curvatus LUHS51, Lactobacillus plantarum LUHS135 and L. curvatus LUHS51, L. plantarum LUHS135 and P. pentosaceus LUHS183 | For wheat bread quality improving (higher porosity, better sensory properties, lower acrylamide concentration) | [20] |
| Lactobacillus coryniformis LUHS71, L. curvatus LUHS51, L. farraginis LUHS206 and Leuconostoc mesenteroides LUHS225 | For wheat bread quality improving (higher porosity, better sensory properties, lower acrylamide concentration); For surface treatment of bread to prolong the shelf life | [22] |
| Pediococcus pentosaceus LUHS183, P. acidilactici LUHS29, Lactobacillus paracasei LUHS244, Lactobacillus brevis LUHS173, Lactobacillus plantarum LUHS135 and Leuconostoc mesenteroides LUHS242 | As antifungal agents against Aspergillus nidulans, Penicillium funiculosum and Fusarium poae; For bread safety improving (lower acrylamide concentration) | [23] |
| Lactobacillus plantarum LUHS135 in combination with savory plants Thymus vulgaris, Carum carvi, Origanum vulgare, Ocimum basilicum and Coriandrum sativum | For bread safety improving (lower acrylamide concentration) | [24] |
| Lacticaseibacillus casei LUHS210 | For almond, coconut and oat drinks by-products valorisation and added-value bread preparation | [25] |
| Lactobacillus paracasei LUHS244 | For okara valorisation and added-value bread preparation | [27] |
| Sourdough LAB Strains | Possible Applications | Reference |
|---|---|---|
| Lactobacillus plantarum, L. uvarum | For wheat production by-products valorisation (safety and nutritional characteristics improving) | [78] |
| Lactobacillus casei strain LUHS210 | For rice, soy, almond, coconut, and oat drinks production by-products valorisation | [76] |
| Pediococcus acidilactici | For barley industry by-products valorisation | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkiene, E.; Özogul, F.; Rocha, J.M. Bread Sourdough Lactic Acid Bacteria—Technological, Antimicrobial, Toxin-Degrading, Immune System-, and Faecal Microbiota-Modelling Biological Agents for the Preparation of Food, Nutraceuticals and Feed. Foods 2022, 11, 452. https://doi.org/10.3390/foods11030452
Bartkiene E, Özogul F, Rocha JM. Bread Sourdough Lactic Acid Bacteria—Technological, Antimicrobial, Toxin-Degrading, Immune System-, and Faecal Microbiota-Modelling Biological Agents for the Preparation of Food, Nutraceuticals and Feed. Foods. 2022; 11(3):452. https://doi.org/10.3390/foods11030452
Chicago/Turabian StyleBartkiene, Elena, Fatih Özogul, and João Miguel Rocha. 2022. "Bread Sourdough Lactic Acid Bacteria—Technological, Antimicrobial, Toxin-Degrading, Immune System-, and Faecal Microbiota-Modelling Biological Agents for the Preparation of Food, Nutraceuticals and Feed" Foods 11, no. 3: 452. https://doi.org/10.3390/foods11030452
APA StyleBartkiene, E., Özogul, F., & Rocha, J. M. (2022). Bread Sourdough Lactic Acid Bacteria—Technological, Antimicrobial, Toxin-Degrading, Immune System-, and Faecal Microbiota-Modelling Biological Agents for the Preparation of Food, Nutraceuticals and Feed. Foods, 11(3), 452. https://doi.org/10.3390/foods11030452