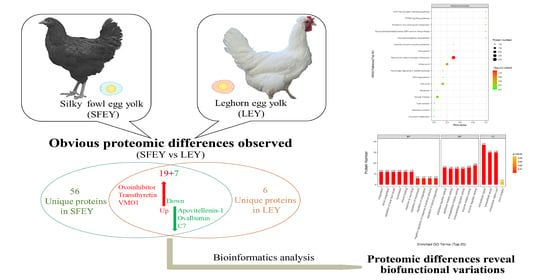
Label-Free LC-MS/MS Analysis Reveals Different Proteomic Profiles between Egg Yolks of Silky Fowl and Ordinary Chickens
Abstract
:1. Introduction
2. Materials and Method
2.1. Preparation of Egg Yolk Samples
2.2. Protein Extraction and Digestion
2.3. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.4. Identification and Quantitation of Proteins
2.5. Bioinformatic Analysis
2.6. Data Availability
3. Results
3.1. Cluster Analysis Showed Obvious Difference of Protein Types and Contents between Two Yolks
3.2. GO Enrichment Analysis Revealed That Peptidase Regulates the Activity of Most Differentially Abundant Proteins Extensively Involved in the Coagulation Process
3.3. KEGG Pathway Analysis Showed Three Differentially Abundant Proteins (Plasminogen, Prothrombin and Angiotensin 1–10) Associated with Neuroactive Ligand–Receptor Interactions Pathway
3.4. Protein–Protein Interaction Analysis Showed Strong Functional Relationships among Three Differentially Abundant Proteins (SERPIN Domain-Containing Protein, Fibrinogen Alpha Chain and Plasminogen)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SFEY | Silky fowl egg yolk |
| LEY | Leghorn egg yolk |
| HTR | 5-hydroxytryptamine receptor 2 |
| TAR | Trace amine-associated receptor |
| AGT | Angiotensinogen II, III |
| AGTR | Angiotensin II receptor type 1 |
| NPFF | Neuropeptide FF-amide peptide |
| NPFFR | Neuropeptide FF receptor 1 |
| NPY | Neuropeptide Y |
| NPYR | Neuropeptide Y receptor |
| AVP | Arginine vasopressin |
| AVPR | Arginine vasopressin receptor 2 |
| PAL | Proteinase-activated like |
| PAR | Proteinase-activated receptor |
| PRLH | Prolactin-releasing-like hormone |
| PRLHR | Prolactin-releasing hormone receptor. |
References
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.K.; Ferreira Júnior, Á.; Zhang, X.Y.; Morgan, P.M. The Domestic Hen. In IgY-Technology: Production and Application of Egg Yolk Antibodies; Zhang, X.Y., Vieira-Pires, R.S., Morgan, P.M., Schade, R., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Su, Y.; Chen, Z.; Li, J.; Chang, C.; Gu, L.; Yang, Y. Characterization of salted egg yolk flavoring prepared by enzyme hydrolysis and microwave irradiation. Food. Chem. 2021, 338, 127913. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Mann, M. The chicken egg yolk plasma and granule proteomes. Proteomics 2008, 8, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Celis, F.; Encinar, J.R.; Sanz-Medel, A. Standardization approaches in absolute quantitative proteomics with mass spectrometry. Mass. Spectrome. Rev. 2018, 37, 715–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sheng, L.; Ma, M.; Jin, Y. Proteome-based identification of chicken egg yolk proteins associated with antioxidant activity on the Qinghai-Tibetan Plateau. Int. J. Biol. Macromol. 2020, 150, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Chen, X.; Geng, Z. Comparative Yolk Proteomic Analysis of Fertilized Low and High Cholesterol Eggs during Embryonic Development. Animals 2021, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Shi, Y.; Ye, H.; Luo, W.; Geng, F. Quantitative proteomic analyses during formation of chicken egg yolk. Food. Chem. 2022, 374, 131828. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.G.; Xie, M.Y.; Wang, W.Y.; Wu, H.J.; Fu, Z.H.; Lin, L. Determination of carnosine in Black-Bone Silky Fowl (Gallus gallus domesticus Brisson) and common chicken by HPLC. Eur. Food. Res. Technol. 2007, 226, 311–314. [Google Scholar] [CrossRef]
- Li, Y.P.; Zhang, X.H.; Huang, J.H.; Huang, H.; Hu, Q.J. Research advances on active peptides from black-bone silky fowl. China Poult. 2017, 39, 38–41. [Google Scholar]
- Zhu, W.Q.; Li, H.F.; Wang, J.Y.; Shu, J.T.; Zhu, C.H.; Song, W.T.; Song, C.; Ji, G.G.; Liu, H.X. Molecular genetic diversity and maternal origin of Chinese black-bone chicken breeds. Genet. Mol. Res. 2014, 13, 3275–3282. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Yue, L.; Jing, X.; Yang, Y.; Xu, Y.; Wu, S.; Liang, Y.; Liu, X.; Zhang, X. Purification and characterization of lysozyme from Chinese Lueyang black-bone Silky fowl egg white. Prep. Biochem. Biotechnol. 2019, 49, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kriangwanich, W.; Piboon, P.; Sakorn, W.; Buddhachat, K.; Kochagul, V.; Pringproa, K.; Mekchay, S.; Nganvongpanit, K. Consistency of dark skeletal muscles in Thai native black-bone chickens (Gallus gallus domesticus). PeerJ 2021, 9, e10728. [Google Scholar] [CrossRef] [PubMed]
- Toyosaki, T.; Koketsu, M. Oxidative stability of silky fowl eggs. Comparison with hen eggs. J. Agric. Food. Chem. 2004, 52, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, M.; Sakuragawa, E.; Linhardt, R.J.; Ishihara, H. Distribution of N-acetylneuraminic acid and sialylglycan in eggs of the Silky fowl. Br. Poult. Sci. 2003, 44, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, L.; Wang, L.; Liu, T.; Wu, X. Proteomic study of Chinese black-bone silky fowl and the ring-necked pheasant egg white by iTRAQ technique. LWT 2021, 150, 111936. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Mann, M. Improved peptide identification in proteomics by two consecutive stages of mass spectrometric fragmentation. Proc. Natl. Acad. Sci. USA 2004, 101, 13417–13422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholewa, B.D.; Pellitteri-Hahn, M.C.; Scarlett, C.O.; Ahmad, N. Large-scale label-free comparative proteomics analysis of polo-like kinase 1 inhibition via the small-molecule inhibitor BI 6727 (Volasertib) in BRAF(V600E) mutant melanoma cells. J. Proteome Res. 2014, 13, 5041–5050. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Zhang, X.; Chelliappan, B.; Somasundaram, R.; Antonysamy, M. Recent Advances in Applications of Bioactive Egg Compounds in Nonfood Sectors. Front. Bioeng. Biotechnol. 2021, 9, 738993. [Google Scholar] [CrossRef]
- Gao, D.; Qiu, N.; Liu, Y.; Ma, M. Comparative proteome analysis of egg yolk plasma proteins during storage. J. Sci. Food Agric. 2017, 97, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.; He, K.; Geng, Z.; Chen, X. Proteomic analysis of fertilized egg yolk proteins during embryonic development. Poult. Sci. 2020, 99, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Réhault, S. Antiproteases. In Bioactive Egg Compounds; Huopalahti, R., López-Fandiño, R., Anton, M., Schade, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 85–92. [Google Scholar]
- Jacobsen, L.; Hermann, M.; Vieira, P.M.; Schneider, W.J.; Nimpf, J. The chicken oocyte receptor for lipoprotein deposition recognizes alpha 2-macroglobulin. J. Biol. Chem. 1995, 270, 6468–6475. [Google Scholar] [CrossRef] [Green Version]
- Moon, K.E.; Gorski, J.P.; Hugli, T.E. Complete primary structure of human C4a anaphylatoxin. J. Biol. Chem. 1981, 256, 8685–8692. [Google Scholar] [CrossRef]
- Dombre, C.; Guyot, N.; Moreau, T.; Monget, P.; Da Silva, M.; Gautron, J.; Réhault-Godbert, S. Egg serpins: The chicken and/or the egg dilemma. Semin. Cell Dev. Biol. 2017, 62, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Werbowetski-Ogilvie, T.E.; Agar, N.Y.; Waldkircher de Oliveira, R.M.; Faury, D.; Antel, J.P.; Jabado, N.; Del Maestro, R.F. Isolation of a natural inhibitor of human malignant glial cell invasion: Inter alpha-trypsin inhibitor heavy chain 2. Cancer Res. 2006, 66, 1464–1472. [Google Scholar] [CrossRef] [Green Version]
- Bourin, M.; Gautron, J.; Berges, M.; Attucci, S.; Le Blay, G.; Labas, V.; Nys, Y.; Rehault-Godbert, S. Antimicrobial potential of egg yolk ovoinhibitor, a multidomain Kazal-like inhibitor of chicken egg. J. Agric. Food Chem. 2011, 59, 12368–12374. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Song, G. Differential expression of vitelline membrane outer layer protein 1: Hormonal regulation of expression in the oviduct and in ovarian carcinomas from laying hens. Mol. Cell. Endocrinol. 2015, 399, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Back, J.F.; Bain, J.M.; Vadehra, D.V.; Burley, R.W. Proteins of the outer layer of the vitelline membrane of hen’s eggs. Biochim. Biophys. Acta 1982, 705, 12–19. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Wu, J. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.N.; Dunn, R.J.; Jeong, S.Y.; Zhu, Q.; Julien, J.P.; David, S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J. Neurosci. 2002, 22, 6578–6586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.J.; Ham, H.S.; Lee, D.S.; Park, H.J. Effects of proteins from hen egg yolk on human platelet aggregation and blood coagulation. Biol. Pharm. Bull. 2003, 26, 1388–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koide, T. Antithrombin and Heparin Cofactor II: Structure and Functions. In Recent Advances in Thrombosis and Hemostasis; Tanaka, K., Davie, E.W., Ikeda, Y., Iwanaga, S., Saito, H., Sueishi, K., Eds.; Springer: Tokyo, Japan, 2008; pp. 177–189. [Google Scholar]
- Colman, R.W. Biologic activities of the contact factors in vivo—Potentiation of hypotension, inflammation, and fibrinolysis, and inhibition of cell adhesion, angiogenesis and thrombosis. Thromb. Haemost. 1999, 82, 1568–1577. [Google Scholar] [PubMed]
- Duan, J.; Yu, Y.; Li, Y.; Li, Y.; Liu, H.; Jing, L.; Yang, M.; Wang, J.; Li, C.; Sun, Z. Low-dose exposure of silica nanoparticles induces cardiac dysfunction via neutrophil-mediated inflammation and cardiac contraction in zebrafish embryos. Nanotoxicology 2016, 10, 575–585. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, A.D.; Liu, M.; Rodríguez, V.; Krause, E.G.; Sumners, C. Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R444–R458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Du, Y.; Boni, G.F.; Liu, X.; Kuang, J.; Geng, Z. Consuming egg yolk decreases body weight and increases serum HDL and brain expression of TrkB in male SD rats. J. Sci. Food Agric. 2019, 99, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.E.; Nunes, A.F.; Sousa, M.M. Transthyretin: More than meets the eye. Prog. Neurobiol. 2009, 89, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.D.; Anders, N.J.; DeCarli, C.; Chan, S.L.; Mattson, M.P.; Johnson, J.A. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: Support for the amyloid hypothesis. J. Neurosci. 2004, 24, 7707–7717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloeckner, S.F.; Meyne, F.; Wagner, F.; Heinemann, U.; Krasnianski, A.; Meissner, B.; Zerr, I. Quantitative analysis of transthyretin, tau and amyloid-beta in patients with dementia. J. Alzheimer’s Dis. 2008, 14, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y. Plasma Transthyretin as a biomarker of Sarcopenia in elderly subjects. Nutrients 2019, 11, 895. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.C.; Bennink, M.R.; Smith, D.M. Composition and functional properties of cholesterol reduced egg yolk. Poult. Sci. 1997, 76, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Sawrey-Kubicek, L.; Zhu, C.; Bardagjy, A.S.; Rhodes, C.H.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption compared with yolk-free egg increases the cholesterol efflux capacity of high-density lipoproteins in overweight, postmenopausal women. Am. J. Clin. Nutr. 2019, 110, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Cornax, I.; Walzem, R.L.; Larner, C.; Macfarlane, R.D.; Klasing, K.C. Mobilization of ectopic yolk in Gallus gallus domesticus: A novel reverse lipid transport process. J. Exp. Biol. 2013, 216, 1949–1958. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, A.C.; Földvári, M.; Spencer, D.F.; Breckenridge, W.C.; Fenwick, R.G.; Williams, D.L.; Mezei, C. The apolipoprotein A-I gene is actively expressed in the rapidly myelinating avian peripheral nerve. J. Cell Biol. 1989, 109, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Bujo, H.; Hermann, M.; Lindstedt, K.A.; Nimpf, J.; Schneider, W.J. Low density lipoprotein receptor gene family members mediate yolk deposition. J. Nutr. 1997, 127, 801s–804s. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Protein Name | Accessions | Unique Peptides | SFEY/LEY Fold Changes | p Value | GO Terms |
|---|---|---|---|---|---|
| Vitelline membrane outer layer protein 1 | P41366 | 1 | 26.452 ↑ | 0.007 | Extracellular region |
| Transthyretin | A0A1L1RWR0 | 3 | 24.708 ↑ | 0.023 | Cellular lipid metabolic process; hormone metabolic process |
| Alpha-2-macroglobulin-like 4 | F1NK40 H1AC38 | 30 | 17.041 ↑ | 0.027 | Molecular function regulator; enzyme regulator activity |
| Ovoinhibitor | P10184 | 18 | 15.797 ↑ | <0.001 | Regulation of proteolysis; peptidase regulator activity; serine-type endopeptidase inhibitor activity |
| Ig-like domain-containing protein | A0A3Q3AN21; A0A3Q2UGI5; A0A3Q2TZD6; A2N890; A2N888 | 2 | 11.154 ↑ | 0.009 | Immune system process |
| C4a anaphylatoxin | Q076D6; D4QB02; D4QB00 | 2 | 8.493 ↑ | <0.001 | Response to stress; proteolysis; peptidase regulator activity |
| Apolipoprotein A-I | P08250 | 10 | 7.843 ↑ | 0.006 | Response to stress; enzyme regulator activity |
| Ig-like domain-containing protein | A0A3Q2U474 | 3 | 7.608 ↑ | <0.001 | Immune system process |
| Ig-like domain-containing protein | A0A1D5PAQ0; A0A3Q2UGD4; A0A3Q2U2P6 | 2 | 6.610 ↑ | 0.003 | Immune system process |
| Avidin-related protein 6-like | A0A3Q2UD25 | 5 | 5.915 ↑ | 0.044 | Amide binding; biotin binding |
| Complement component 3 | A0A1D5P9F9; A0A1D5PWR4; A0A1D5PFG3; A0A1D5PQ85; A6N9E0; Q2MV09; Q6PPA9 | 10 | 5.416 ↑ | <0.001 | Regulation of response to external stimulus; regulation of proteolysis; peptidase regulator activity; endopeptidase regulator activity |
| Kininogen 1 | A0A1L1RNR4 | 5 | 5.060 ↑ | <0.001 | Hemostasis; blood coagulation; peptidase regulator activity |
| Complement factor H | A0A3Q2TRY3A0A3 Q2TXN4E1C7P4 A0A3Q3AX54 A0A3Q3AK26 | 25 | 4.301 ↑ | 0.042 | Immune effector process; protein activation cascade |
| C4a anaphylatoxin | A0A290WNG2 | 3 | 3.694 ↑ | 0.035 | Response to stress; proteolysis; peptidase regulator activity |
| Prothrombin | Q91001; F1NXV6 | 11 | 3.534 ↑ | 0.006 | Hemostasis; blood coagulation; serine-type endopeptidase activity |
| Ig-like domain-containing protein | A0A3Q2U343 | 1 | 2.831 ↑ | 0.018 | Immune system process |
| Plasminogen | F1NWX6; Q7LZF3 | 24 | 2.816 ↑ | 0.002 | Hemostasis; blood coagulation; serine-type endopeptidase activity |
| PIT 54 | Q98TD1; A0A1L1S0P1 | 13 | 2.651 ↑ | <0.001 | Scavenger receptor activity |
| Fibrinogen alpha chain | P14448; F1P4V1 | 13 | 2.527 ↑ | 0.043 | Hemostasis; blood coagulation; response to stress |
| Ig-like domain-containing protein | A0A3Q2UAA5 | 7 | 0.413 ↓ | <0.001 | Immune system process |
| Dishevelled binding antagonist of beta catenin 2 | A0A1D5NWD7 | 1 | 0.380 ↓ | 0.002 | Negative regulation of cell adhesion; regulation of response to stimulus; tube development |
| Ig-like domain-containing protein | A0A3Q2U9M3 | 2 | 0.373 ↓ | 0.008 | Immune system process |
| Apovitellenin-1 | P02659 | 7 | 0.248 ↓ | <0.001 | Lipid metabolic process; enzyme inhibitor activity |
| Ovalbumin (Fragment) | A0A2H4Y866; A0A2H4Y8E8; P0102; A0A2H4Y8S8; A0A2H4Y8R9; A0A2H4Y8R6; A0A2H4Y8R1 | 6 | 0.233 ↓ | 0.001 | Extracellular region |
| Complement component 7 | F1DQG4; E1C6U2 | 17 | 0.19 ↓ | <0.001 | Immune effector process; protein activation cascade |
| Avidin | E1BTQ4; A0A3Q2U4N2; A0A3Q2UC51 | 3 | 0.113 ↓ | 0.003 | Amide binding; biotin binding |
| Domain Name | GO Category | ProteinsVar Number | ProteinsVar Name |
|---|---|---|---|
| Serpin (serine protease inhibitor) | / | 8 | SERPIN domain-containing protein (A0A1D5PI58, E1C7T1, F1NAR5), Heparin cofactor II (Fragment), Ovalbumin-related protein Y, Angiotensin 1–10, Antithrombin-III (Fragment), Ovalbumin (A0A1D5PI58) |
| Alpha-2-macroglobulin family | / | 6 | Alpha-2-macroglobulin-like 4, C4a anaphylatoxin (Q07606, A0A290WNG2), Complement component 3, Complement component 5, Uncharacterized protein (A0A3Q3B2L3) |
| Alpha-2-macroglobulin family N-terminal region | / | 6 | Alpha-2-macroglobulin-like 4, C4a anaphylatoxin (Q07606, A0A290WNG2), Complement component 3, Complement component 5, Uncharacterized protein (A0A3Q3B2L3) |
| Anaphylotoxin-like domain | / | 5 | C4a anaphylatoxin (Q07606, A0A290WNG2), Complement component 3, Complement component 5, Fibulin-1 |
| Vitamin K-dependent carboxylation/ gamma-carboxyglutamic (GLA) domain | / | 4 | Prothrombin, Vitamin K-dependent protein S, Anticoagulant protein C, Coagulation factor X |
| MG2 domain | / | 5 | Alpha-2-macroglobulin-like 4, C4a anaphylatoxin (Q07606, A0A290WNG2), Complement component 3, Complement component 5 |
| Kringle domain | / | 3 | Prothrombin, Plasminogen, Coagulation factor XII |
| Trypsin | / | 5 | Prothrombin, Plasminogen, Coagulation factor XII, Anticoagulant protein C, Coagulation factor X |
| A-macroglobulin receptor | / | 5 | Alpha-2-macroglobulin-like 4, C4a anaphylatoxin (Q07606, A0A290WNG2), Complement component 3, Complement component 5 |
| A-macroglobulin complement component | / | 5 | Alpha-2-macroglobulin-like 4, C4a anaphylatoxin (Q07606, A0A290WNG2), Complement component 3, Complement component 5 |
| / | Peptidase regulator activity, peptidase inhibitor activity, endopeptidase inhibitor activity, endopeptidase regulator activity, enzyme inhibitor activity. | 15 | SERPIN domain-containing protein (E1C7T1, F1NAR5), Heparin cofactor II (Fragment), Ovalbumin-related protein Y, Angiotensin 1–10, Antithrombin-III (Fragment), Ovoinhibitor, Alpha-2-macroglobulin-like 4, C4a anaphylatoxin (Q07606, A0A290WNG2), Complement component 3, Complement component 5, Kininogen-1, Inter-alpha inhibitor heavy chain 2, Uncharacterized protein (A0A3Q3B2L3) |
| / | Serine-type endopeptidase activity. | 5 | Prothrombin, Plasminogen, Coagulation factor XII, Anticoagulant protein C, Coagulation factor X |
| / | Hemostasis, coagulation, blood coagulation, regulation of body fluid levels, wound healing, response to wounding. | 12 | Prothrombin, Plasminogen, Coagulation factor XII, Anticoagulant protein C, Coagulation factor X, Vitamin K-dependent protein S, Fibrinogen alpha chain, Kininogen-1, Peptidase_M14 domain-containing protein, SERPIN domain-containing protein (F1NAR5), Antithrombin-III (Fragment), Heparin cofactor II (Fragment) |
| / | Regulation of response to wounding, regulation of wound healing, regulation of blood coagulation, regulation of coagulation, regulation of hemostasis | 6 | Kininogen-1, Vitamin K-dependent protein S, Plasminogen, Peptidase_M14 domain-containing protein, SERPIN domain-containing protein (F1NAR5), Antithrombin-III (Fragment) |
| / | Extracellular region, extracellular space, extracellular region part | 30 | SERPIN domain-containing protein (A0A1D5PI58, E1C7T1, F1NAR5), Heparin cofactor II (Fragment), Ovalbumin-related protein Y, Angiotensin 1–10, Antithrombin-III (Fragment), Ovalbumin (A0A1D5PI58), Alpha-2-macroglobulin-like 4, C4a anaphylatoxin (Q07606, A0A290WNG2), Complement component 3, Complement component 5, Fibrinogen alpha chain, 60 kDa heat shock protein, Apovitellenin-1, Apolipoprotein A-I, Gallinacin-9, Ceruloplasmin, Glycosyl-phosphatidylinositol-specific phospholipase D, Kininogen-1, Transthyretin, Alpha-1-acid glycoprotein, Coagulation factor XII, Peptidase_M14 domain-containing protein, Ovoinhibitor, Coagulation factor X, 14-3-3 protein zeta, Vitelline membrane outer layer protein 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Chen, C.; Zhang, X. Label-Free LC-MS/MS Analysis Reveals Different Proteomic Profiles between Egg Yolks of Silky Fowl and Ordinary Chickens. Foods 2022, 11, 1035. https://doi.org/10.3390/foods11071035
Wu R, Chen C, Zhang X. Label-Free LC-MS/MS Analysis Reveals Different Proteomic Profiles between Egg Yolks of Silky Fowl and Ordinary Chickens. Foods. 2022; 11(7):1035. https://doi.org/10.3390/foods11071035
Chicago/Turabian StyleWu, Rao, Chen Chen, and Xiaoying Zhang. 2022. "Label-Free LC-MS/MS Analysis Reveals Different Proteomic Profiles between Egg Yolks of Silky Fowl and Ordinary Chickens" Foods 11, no. 7: 1035. https://doi.org/10.3390/foods11071035
APA StyleWu, R., Chen, C., & Zhang, X. (2022). Label-Free LC-MS/MS Analysis Reveals Different Proteomic Profiles between Egg Yolks of Silky Fowl and Ordinary Chickens. Foods, 11(7), 1035. https://doi.org/10.3390/foods11071035