Antiradical Potential of Food Products as a Comprehensive Measure of Their Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Materials
2.2. Extract Preparations
2.3. ARP Measurement
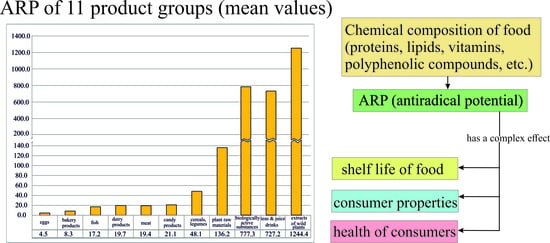
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popkin, B.M. Urbanization, lifestyle changes and the nutrition transition. World Dev. 1999, 27, 1905–1916. [Google Scholar] [CrossRef]
- Steele, R. (Ed.) Understanding and Measuring the Shelf-Life of Food; CRC Press: Boca Raton, FL, USA, 2004; p. 407. [Google Scholar] [CrossRef]
- Ottaway, P.B. (Ed.) Food Fortification and Supplementation: Technological, Safety and Regulatory Aspects; CRC Press: Boca Raton, FL, USA, 2008; p. 252. [Google Scholar] [CrossRef]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The role of food antioxidants, benefits of functional foods, and Influence of feeding habits on the health of the older person: An overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Li, H. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Corvo, P. Biosocial risks of food: International aspects. MGIMO Rev. Int. Rel. 2017, 1, 67–82. (In Russian) [Google Scholar] [CrossRef]
- Popkin, B. Ultra-Processed Foods’ Impacts on Health. 2030—Food, Agriculture and Rural Development in Latin America and the Caribbean; FAO: Santiago, Chile, 2020; Volume 34, p. 26. [Google Scholar]
- Clark, M.A.; Springmann, M.; Hill, J.; Tilman, D. Multiple health and environmental impacts of foods. Proc. Natl. Acad. Sci. USA 2019, 116, 23357–23362. [Google Scholar] [CrossRef] [Green Version]
- Delang, C.O. Causes and distribution of soil pollution in China. Environ. Socio-Econ. Stud. 2017, 5, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef]
- White, A.P.; Handler, E.; Smith, R.; Hill, R.; Lehman, M.G. Principles of Biochemistry; McGraw-Hill: New York, NY, USA; Michigan University: Ann Arbor, MI, USA, 1978; p. 1492. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Bidar, A.K.; Ragashtai, A.R. Factors influencing consumer preference for purchasing of processed food products in modern retail formats—A study in bengaluru city. Environ. Ecol. 2019, 37, 1–4. [Google Scholar]
- Ares, G.; Vidal, L.; Allegue, G.; Giménez, A.; Bandeira, E.; Moratorio, X.; Curutchet, M.R. Consumers’ conceptualization of ultra-processed foods. Appetite 2016, 105, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Nemkova, E.V. Factors and types of consumer behavior in the food market. Electron. J. Econ. Soc. 2008, 9, 68–85. (In Russian) [Google Scholar]
- Kalugina, I.Y.; Gorina, D.N. Formation of health-saving thinking among food industry specialists. In Innovative technologies in the food industry and public catering. In Proceedings of the 6th International Scientific and Practical Conference of Food Technology, Ekaterinburg, Russia, 16 April 2019; pp. 57–63. (In Russian). [Google Scholar]
- Timofeeva, V.A. Merchandising of Food Products; Phoenix: Rostov-on-Don, Russia, 2013; p. 494. (In Russian) [Google Scholar]
- Glavind, J. Antioxidants in animal tissue. Acta. Chem. Scand. 1963, 17, 1635–1640. [Google Scholar] [CrossRef]
- Lapinskii, A.G.; Gorbachev, V.V. The antiradical activity of extracts from some wild-growing plants of the Okhotsk Sea northern coastal region. Pharm. Chem. J. 2006, 40, 317–319. [Google Scholar] [CrossRef]
- Kutsenko, S.A. Fundamentals of Toxicology: Scientific-Methodical Edition; Foliant: Saint Petersburg, Russia, 2004; p. 637. [Google Scholar]
- Jukes, T.H.; Schmidt, C.L.A. The apparent dissociation constants of certain amino acids and related substances in water-ethanol mixtures. J. Biol. Chem. 1934, 105, 359–371. [Google Scholar] [CrossRef]
- Nozaki, Y.; Tanford, C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. J. Biol. Chem. 1971, 246, 2211–2217. [Google Scholar] [CrossRef]
- Gulcin, I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007, 32, 431–438. [Google Scholar] [CrossRef]
- Bowden, N.A.; Sanders, J.P.M.; Bruins, M.E. Solubility of the proteinogenic α-amino acids in water, ethanol, and ethanolwater mixtures. J. Chem. Eng. Data 2018, 63, 488–497. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef] [Green Version]
- Amarowicz, R. Antioxidant activity of protein hydrolysates. Eur. J. Lipid Sci. Technol. 2008, 110, 489–490. [Google Scholar] [CrossRef]
- Karaulova, E.P.; Chepkasova, A.I.; Slutskaya, T.N.; Shulgina, L.V.; Yakush, E.V. Antiradical effect of low-molecular peptides in extracts and hydrolyzates from tissues of water organisms. Izv. TINRO 2015, 182, 269–276. (In Russian) [Google Scholar] [CrossRef]
- Widomska, J.; Raguz, M.; Subczynski, W.K. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 2635–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serbinova, E.A.; Packer, L. Antioxidant properties of α-tocopherol and α-tocotrienol. Methods Enzymol. 1994, 234, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Theile, K.; Böhm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Collection of Recipes for Bakery Products Produced According to State Standards; GIORD: Saint Petersburg, Russia, 2004; p. 92. (In Russian)
- Fomenko, S.E.; Kushnerova, N.F.; Sprygin, V.G.; Drugova, E.S.; Lesnikova, L.N.; Merzlyakov, V.Y.; Momot, T.V. Lipid composition, content of polyphenols, and antiradical activity in some representative of marine algae. Russ. J. Plant Phys. 2019, 66, 942–949. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food. Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef]
- Bykov, D.E.; Makarova, N.V.; Valiulina, D.F. Chocolate as a product for functional nutrition. Vestn. MGTU 2018, 21, 447–459. [Google Scholar] [CrossRef]
- Skurikhin, I.M.; Tutelyan, V.A. Chemical Composition of Russian Food Products: A Reference Book; DeLi Print: Moscow, Russia, 2002; p. 236. (In Russian) [Google Scholar]
- Delang, C.O. The consequences of soil degradation in China: A review. GeoScape 2018, 12, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Gileva, A.T.; Myasnikova, E.B. Economic growth in agriculture in China. Bull. Tula Bran. Fin. Univ. 2018, 1, 62–64. (In Russian) [Google Scholar]
- Deineka, V.I.; Deineka, L.A.; Miachikova, N.I.; Sorokopudov, V.N.; Sorokopudova, O.; Nazarenko, S.M. Plant Fruits Anthocyanins of the Belgorod Region. Adv. Environ. Biol. 2014, 8, 540–543. [Google Scholar]
- Sarafanova, L.A. The Use of Food Additives in the Beverage Industry; Profession: Saint Petersburg, Russia, 2007; p. 249. [Google Scholar]
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and antiradical activity of coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Nikolayeva, T.N.; Zagoskina, N.V.; Malyukova, L.S. Antiradical activity in aqueous extracts from tea plants, grown under different conditions of mineral nutrition. Subtr. Décor. Garden. 2018, 64, 111–115. [Google Scholar]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł. Black tea samples origin discrimination using analytical investigations of secondary metabolites, antiradical scavenging activity and chemometric approach. Molecules 2018, 23, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waszkowiak, K.; Mikołajczak, B. The Effect of Roasting on the Protein Profile and Antiradical Capacity of Flaxseed Meal. Foods 2020, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Buldakov, A.S. Food Additives; Reference; Ut: Saint Petersburg, Russia, 1996; p. 240. (In Russian) [Google Scholar]
- Dotsenko, S.M.; Skripko, O.V.; Statsenko, E.S. Culinary products based on combined fish mince. New. High. Edu. Inst. Food Technol. 2006, 1, 63–66. (In Russian) [Google Scholar]
- Battinelli, L.; Tita, B.; Evandri, M.G.; Mazzanti, G. Antimicrobial activity of Epilobium spp. Extracts. Il Farmaco 2001, 56, 345–348. [Google Scholar] [CrossRef]
- Ishisaka, A.; Mukai, R.; Terao, J.; Shibata, N.; Kawai, Y. Specific localization of quercetin-3-O-glucuronide in human brain. Arch. Biochem. Biophys. 2014, 557, 11–17. [Google Scholar] [CrossRef]
| Product Name | Equivalents (95% CI) |
|---|---|
| Hemp hardtacks with the addition of hemp flour (Russian Federation) | 3.05–3.40 |
| Wheat bread “Baton” (long loaf) (Russian Federation) | 4.02–5.34 |
| Bread made from a mixture of rye and wheat flour (Russian Federation) | 4.61–5.14 |
| Bread with cranberries “Tayozhny” (Russian Federation) | 8.48–8.93 |
| Rye bread “Borodinsky” (Russian Federation) | 18.53–21.70 |
| Mean value: | 7.74–8.90 |
| Substance/Preparation Name | Equivalents (95% CI) |
|---|---|
| Monosodium glutamate crystalline (China) | 0.90–1.96 |
| Pea protein isolate (protein concentration 75%) (Great Britain) | 14.37–16.48 |
| Hydrolyzed whey protein (protein concentration 80%) (USA) | 29.15–30.48 |
| Soy protein isolate (protein concentration 90%) (Great Britain) | 34.97–40.07 |
| Arginine powder (USA) | 52.33–65.65 |
| Complex preparation of essential amino acid in powder Amino-9 (leucine, lysine, phenylalanine, valine, threonine, isoleucine, methionine, histidine, tryptophan) (USA) | 93.9–106.7 |
| Acetylcysteine powder (NAC) with Se (USA) | 7474.19–9166.13 |
| Complex vitamin preparation of tocopherols (the main α-form with an admixture of β- γ- δ-. without the addition of tocotrienols) (USA) | 12.17–15.78 |
| Soy lecithin (in terms of phosphatidylinositols in ≈20% by weight of lecithin) (USA) | 16.3–17.8(81.72–88.90) |
| Conjugated Linoleic Acid—CLA (USA) | 29.72–33.82 |
| DHA+ EPA—docosahexaenoic and eicosapentaenoic acids in the ratio 2:1 (USA) | 40.23–40.82 |
| Chlorophyll extract liquid (in terms of pure chlorophyll) (USA) | 92.01–100.48(1945.26–2203.45) |
| Lipoic Acid Powder (USA) | 119.12–123.98 |
| Selenic acid (Russian Federation) | 300.50–306.30 |
| Complex vitamin preparation B-100—B1; B2; B6; B12; niacin; folate; biotin; pantothenate (USA) | 870.38–1390.46 |
| Milk thistle bioflavonoids with the addition of extracts from dandelion and artichokes in a ratio of 6:2:1 (USA) | 2728.16–3137.53 |
| Mean value: | 706.84–847.77 |
| Product Name | Equivalents (95% CI) |
|---|---|
| Caramel made from pure beet sugar (own production) | 2.26–3.26 |
| “Darletto” soft waffles with whipped cream and blueberries (Russian Federation) | 4.76–5.17 |
| Pink chocolate with strawberry flavor (Belgium, Callebaut) | 8.74–9.14 |
| “Fruit nectar” Marmalade slices with mango flavor (Russian Federation) | 9.25–9.69 |
| “Druzhba” Halva with peanuts (Russian Federation) | 9.32–9.57 |
| Milk chocolate (Belgium, Callebaut) | 11.61–11.81 |
| “Babaevskiy” Dark chocolate (Russian Federation) | 41.74–43.91 |
| “Rossiyskiy” cocoa powder (Russian Federation) | 52.46–54.47 |
| Non-sulfated cane molasses (Paraguay, Wholesome Sweeteners Inc.) | 44.68–47.58 |
| Mean value: | 20.54–21.64 |
| Product Name | Equivalents (95% CI) |
|---|---|
| Rice (Russian Federation) | 7.96–23.61 |
| Chickpea beans (India) | 11.11–14.91 |
| Amaranth (India) | 14.20–23.12 |
| Quinoa (Ecuador) | 22.92–26.24 |
| Lentil beans (India) | 48.57–49.72 |
| Ground flax seeds, fat-free (Russian Federation) | 48.36–58.60 |
| Buckwheat (Russian Federation) | 63.91–67.25 |
| Chia seeds (Mexico) | 82.59–91.04 |
| Mung beans (China) | 98.04–113.33 |
| Mean value: | 44.18–51.98 |
| Product Name | Equivalents (95% CI) |
|---|---|
| Dried mushrooms—Leophyllum decastes (Russian Federation) | 17.39–18.65 |
| Chinese cabbage, fresh (China) | 46.34–57.28 |
| Broccoli, fresh-frozen (Poland) | 58.94–65.43 |
| Fresh-frozen porcini mushrooms (Poland) | 58.98–61.58 |
| Fresh-frozen honey mushrooms (China) | 74.30–84.97 |
| Fresh-frozen champignons mushrooms (China) | 130.92–132.25 |
| Fresh Ligol apples (Republic of Moldova) | 168.18–172.29 |
| Cherry, fresh-frozen (Poland) | 473.53–563.00 |
| Mean value: | 128.10–144.21 |
| Mean value (for vegetable raw materials without mushrooms): | 187.29–215.52 |
| Mean value (only for mushrooms): | 70.40–74.36 |
| Product Name | Equivalents (95% CI) |
|---|---|
| Carbonated drink “Mangosteen” (Russian Federation) | 6.49–6.55 |
| Juice drink “Apple” (Russian Federation) | 21.09–25.35 |
| Juice drink “Multi-fruit” (Russian Federation) | 37.43–37.89 |
| Juice drink “Apple/grape” (Russian Federation) | 57.09–60.94 |
| Hibiscus drink (Egypt) | 601.68–665.46 |
| Hibiscus drink (Russian Federation) | 645.61–666.50 |
| Medium roasted “Coffee Jockey” (Russian Federation) | 836.67–842.86 |
| Black tea “Brooke Bond” (Netherlands) | 2045.08–2134.17 |
| Green tea “Greenfield Flying Dragon” (Russian Federation) | 2132.79–2265.9 |
| Mean value: | 709.32–745.07 |
| Product Name | Equivalents (95% CI) |
|---|---|
| Raw cow’s milk (Russian Federation) | 3.42–4.19 |
| Cheese “Light” (Republic of Belarus) | 4.92–8.12 |
| Cheese “Dorblu” (Russian Federation) | 8.07–10.35 |
| Boiled cow’s milk (Russian Federation) | 18.16–20.51 |
| Yogurt without sugar, without fruit additives (Russian Federation) | 54.07–65.02 |
| Mean value: | 17.73–21.64 |
| Product Name | Equivalents (95% CI) |
|---|---|
| Canned food: stewed pork, without fat (Russian Federation) | 10.60–12.08 |
| Baked chicken meat (Russian Federation) | 12.09–13.68 |
| Fresh-frozen chicken meat (Russian Federation) | 14.72–22.54 |
| “Odesskaya” sausage (Russian Federation) | 34.58–35.05 |
| Mean value: | 18.00–20.84 |
| Mean value (without sausage): | 12.47–16.10 |
| Product Name | Equivalents (95% CI) |
|---|---|
| Pollock mince, fresh-frozen (Russian Federation) | 1.45–3.96 |
| Canned herring (Russian Federation) | 2.35–3.15 |
| Pollock, boiled (Russian Federation) | 4.11–5.04 |
| Pollock, fresh-frozen (Russian Federation) | 4.20–6.08 |
| Canned pink salmon (Russian Federation) | 6.22–12.51 |
| Tilapia, fresh-frozen (Thailand) | 9.31–13.72 |
| Tilapia, boiled (Thailand) | 11.55–13.50 |
| Pink salmon, fresh-frozen (Russian Federation) | 11.81–13.95 |
| Pacific herring, fresh-frozen (Russian Federation) | 12.36–14.69 |
| Chum salmon, fresh-frozen (Russian Federation) | 14.20–15.95 |
| Salmon milt, boiled (Russian Federation) | 19.33–21.21 |
| Navaga, fresh-frozen (Russian Federation) | 22.98–24.66 |
| Northern shrimps, boiled, fresh-frozen (Denmark) | 24.82–25.88 |
| Canned saury (Russian Federation) | 26.47–31.03 |
| Canned tuna (Thailand) | 27.13–30.20 |
| Salmon milt, fresh-frozen (Russian Federation) | 45.12–48.10 |
| Mean value: | 15.91–18.54 |
| Mean value (for fresh-frozen raw materials) | 15.32–17.88 |
| (excluding pollock): | (20.32–23.02) |
| Mean value (for thermally processed products): | 16.43–19.11 |
| Name of Wild Plants (Latine) and Production Parameters | Equivalents (95% CI) |
|---|---|
| Dahurian larch (Larix gmelinii), extracts from fine and coarse wood fractions | 194.61–198.91 |
| Extracts from powder preparations of brown, red, and green algae of the Sea of Okhotsk (genus: Laminaria, Alaria, Ulva, Fucus, Chondrus, Neohypophyllum, Porphyra). Average ARP values | 213.2–237.81 |
| Siberian dwarf pine (Pinus pumila), average values from extracts of dried needles, apical parts, and extracts from fresh samples | 501.96–544.80 |
| Rosebay willowherb, additional drying at 150 °C, 4 h (Chamaenerion angustifolium) | 782.45–792.52 |
| Marigolds (Tagetes patula), petal extract | 931.12–978.24 |
| Rosebay willowherb (Chamaenerion angustifolium), average values for extracts from leaves and flowers, drying at 60 °C | 2611.12–2681.02 |
| Rosebay willowherb (Chamaenerion angustifolium), local producers (Seymchan settlement, Magadan region) | 3313.95–3440.03 |
| Mean value: | 1221.20–1267.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbachev, V.; Klokonos, M.; Mutallibzoda, S.; Tefikova, S.; Orlovtseva, O.; Ivanova, N.; Posnova, G.; Velina, D.; Zavalishin, I.; Khayrullin, M.; et al. Antiradical Potential of Food Products as a Comprehensive Measure of Their Quality. Foods 2022, 11, 927. https://doi.org/10.3390/foods11070927
Gorbachev V, Klokonos M, Mutallibzoda S, Tefikova S, Orlovtseva O, Ivanova N, Posnova G, Velina D, Zavalishin I, Khayrullin M, et al. Antiradical Potential of Food Products as a Comprehensive Measure of Their Quality. Foods. 2022; 11(7):927. https://doi.org/10.3390/foods11070927
Chicago/Turabian StyleGorbachev, Victor, Maria Klokonos, Sherzodkhon Mutallibzoda, Svetlana Tefikova, Olga Orlovtseva, Natalia Ivanova, Galina Posnova, Daria Velina, Igor Zavalishin, Mars Khayrullin, and et al. 2022. "Antiradical Potential of Food Products as a Comprehensive Measure of Their Quality" Foods 11, no. 7: 927. https://doi.org/10.3390/foods11070927
APA StyleGorbachev, V., Klokonos, M., Mutallibzoda, S., Tefikova, S., Orlovtseva, O., Ivanova, N., Posnova, G., Velina, D., Zavalishin, I., Khayrullin, M., Bobkova, E., Kuznetsova, E., Vorobeva, A., Vorobyev, D., & Nikitin, I. (2022). Antiradical Potential of Food Products as a Comprehensive Measure of Their Quality. Foods, 11(7), 927. https://doi.org/10.3390/foods11070927