Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms
Abstract
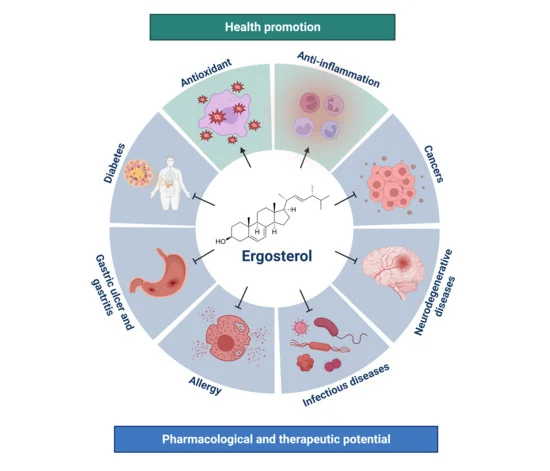
:1. Introduction
2. Overview of Ergosterol Structure
3. Natural Sources of Ergosterol
4. Pharmacokinetics, Drug-Likeness, and Toxicity of Ergosterol
5. Antioxidant Activity
6. Anti-Inflammatory Activity
7. Anticancer Activity
| Cancer | Model | Dose | Activity and Mechanism | Reference |
|---|---|---|---|---|
| Bladder | N-butyl-N-(4-hydroxybutyl)nitrosamine-induced bladder cancer in Wistar rats | 15 μg/kg/day for 3 weeks | Modulate inflammation-related signaling and inhibit androgen signaling pathways | [59] |
| A diet which contains ergosterol 0.01–0.1% for 25 weeks | Inhibit androgen signaling | [83] | ||
| Breast | Carcinogen-induced normal breast cell lines MCF10A and MCF12A | 1–50 µM | Block carcinogen-induced ROS, ERK activation, DNA oxidation, and DNA damage. | [79] |
| MCF7 breast cancer cell lines | IC50 = 112.65 μM | Induce S-phase cell cycle arrest and apoptosis | [77,78] | |
| Liver | Hep3B and HepJ5 human hepatocellular carcinoma cells | IC50 of Hep3B and HepJ5 cells from 14.54–6.66 μM and 18.65–4.07 μM, respectively, when combined with amphotericin B (5–25 μM) | Increase ROS and LC3-II levels | [81] |
| Prostrate | LNCaP human prostate adenocarcinoma cell | IC50 = 14.68 ± 1.01 μM | Inhibit androgen receptor | [84] |
| Sarcoma | Sarcoma 180-bearing mice | 400 and 800 mg/kg for 20 days | N/A | [85] |
| Tumor | Matrigel-induced neovascularization in C57BL/6 mice | 5, 10 and 20 mg/kg for 5 days | Inhibit angiogenesis | [85] |
8. Antidiabetic Effects
9. Neuroprotective Effects
10. Antimicrobial Activity
11. Anti-Hepatic Steatosis Effect
12. Future Perspective and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bell, V.; Silva, C.; Guina, J.; Fernandes, T.H. Mushrooms as future generation healthy foods. Front. Nutr. 2022, 9, 1050099. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.B.; Brown, A.J. A detour to sterol synthesis. Nat. Microbiol. 2019, 4, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, Y.; Matsuki, K.; Iijima, T.; Nakano, S.; Kakuda, R.; Machida, K.; Kikuchi, M. New sterols and triterpenoids from four edible mushrooms. Chem. Pharm. Bull. 2001, 49, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haubrich, B.A. Microbial Sterolomics as a Chemical Biology Tool. Molecules 2018, 23, 2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villares, A.; García-Lafuente, A.; Guillamón, E.; Ramos, Á. Identification and quantification of ergosterol and phenolic compounds occurring in Tuber spp. truffles. J. Food Compos. Anal. 2012, 26, 177–182. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–757; quiz 757–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quackenbush, F.W.; Peterson, W.H.; Steenbock, H. A Study of the Nutritive Value of Mushrooms: Five Figures. J. Nutr. 1935, 10, 625–643. [Google Scholar] [CrossRef]
- Shao, S.; Hernandez, M.; Kramer, J.K.; Rinker, D.L.; Tsao, R. Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J. Agric. Food Chem. 2010, 58, 11616–11625. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a Novel Methodology for the Analysis of Ergosterol in Mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT—Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Belwal, T.; Li, L.; Luo, Z. Phytosterols extraction from hickory (Carya cathayensis Sarg.) husk with a green direct citric acid hydrolysis extraction method. Food Chem. 2020, 315, 126217. [Google Scholar] [CrossRef]
- Ghisoni, S.; Lucini, L.; Rocchetti, G.; Chiodelli, G.; Farinelli, D.; Tombesi, S.; Trevisan, M. Untargeted metabolomics with multivariate analysis to discriminate hazelnut (Corylus avellana L.) cultivars and their geographical origin. J. Sci. Food Agric. 2020, 100, 500–508. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Hamed, M.S.; Khiralla, G.M.; Mohamed, A.F. Cactus and lupin extracts as prospective anticancer agents compared with utoral drug. J. Food Biochem. 2020, 44, e13299. [Google Scholar] [CrossRef] [PubMed]
- Keser, S. Antiradical activities and phytochemical compounds of firethorn (Pyracantha coccinea) fruit extracts. Nat. Prod. Res. 2014, 28, 1789–1794. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Siwulski, M.; Wiater, A.; Komaniecka, I.; Komosa, A.; Gąsecka, M.; Magdziak, Z.; Mleczek, M.; Niedzielski, P.; Proch, J.; et al. The Effect of Mushroom Extracts on Human Platelet and Blood Coagulation: In vitro Screening of Eight Edible Species. Nutrients 2019, 11, 3040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa, R.C.G.; Barros, L.; Fernandes, Â.; Sokovic, M.; Bracht, A.; Peralta, R.M.; Ferreira, I. A natural food ingredient based on ergosterol: Optimization of the extraction from Agaricus blazei, evaluation of bioactive properties and incorporation in yogurts. Food Funct. 2018, 9, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Sillapachaiyaporn, C.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S. Anti-HIV-1 protease activity of the crude extracts and isolated compounds from Auricularia polytricha. BMC Complement. Altern. Med. 2019, 19, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sillapachaiyaporn, C.; Rangsinth, P.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S.; Tencomnao, T. Neuroprotective Effects against Glutamate-Induced HT-22 Hippocampal Cell Damage and Caenorhabditis elegans Lifespan/Healthspan Enhancing Activity of Auricularia polytricha Mushroom Extracts. Pharmaceuticals 2021, 14, 1001. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide—UHPLC-MS/MS analysis. Food Chem. 2022, 369, 130927. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Li, R.; Wu, X.; Zheng, C.; Shiu, P.H.-T.; Chan, J.C.-K.; Rangsinth, P.; Liu, C.; Leung, S.W.-S.; et al. Protective Effects of Amauroderma rugosum on Doxorubicin-Induced Cardiotoxicity through Suppressing Oxidative Stress, Mitochondrial Dysfunction, Apoptosis, and Activating Akt/mTOR and Nrf2/HO-1 Signaling Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 9266178. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of Biologically Active Ganoderma lucidum Compounds and Synthesis of Improved Derivatives That Confer Anti-cancer Activities in vitro. Front. Pharm. 2019, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Kawai, J.; Mori, K.; Hirasawa, N. Grifola frondosa extract and ergosterol reduce allergic reactions in an allergy mouse model by suppressing the degranulation of mast cells. Biosci. Biotechnol. Biochem. 2019, 83, 2280–2287. [Google Scholar] [CrossRef]
- Kawai, J.; Higuchi, Y.; Hirota, M.; Hirasawa, N.; Mori, K. Ergosterol and its derivatives from Grifola frondosa inhibit antigen-induced degranulation of RBL-2H3 cells by suppressing the aggregation of high affinity IgE receptors. Biosci. Biotechnol. Biochem. 2018, 82, 1803–1811. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.G.; Bishop, K.S.; Tanambell, H.; Buchanan, P.; Smith, C.; Quek, S.Y. Characterization of the bioactivities of an ethanol extract and some of its constituents from the New Zealand native mushroom Hericium novae-zealandiae. Food Funct. 2019, 10, 6633–6643. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 55, 378–385. [Google Scholar] [CrossRef]
- Morales, D.; Tejedor-Calvo, E.; Jurado-Chivato, N.; Polo, G.; Tabernero, M.D.; Ruíz-Rodríguez, A.; Largo, C.; Soler-Rivas, C.J.F. In vitro and in vivo testing of the hypocholesterolemic activity of ergosterol- and β-glucan-enriched extracts obtained from shiitake mushrooms (Lentinula edodes). Food Funct. 2019, 10, 7325–7332. [Google Scholar] [CrossRef] [PubMed]
- Drori, A.; Shabat, Y.; Ben Ya’acov, A.; Danay, O.; Levanon, D.; Zolotarov, L.; Ilan, Y. Extracts from Lentinula edodes (Shiitake) Edible Mushrooms Enriched with Vitamin D Exert an Anti-Inflammatory Hepatoprotective Effect. J. Med. Food 2016, 19, 383–389. [Google Scholar] [CrossRef]
- Quintero-Cabello, K.P.; Palafox-Rivera, P.; Lugo-Flores, M.A.; Gaitán-Hernández, R.; González-Aguilar, G.A.; Silva-Espinoza, B.A.; Tortoledo-Ortiz, O.; Ayala-Zavala, J.F.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A. Contribution of Bioactive Compounds to the Antioxidant Capacity of the Edible Mushroom Neolentinus lepideus. Chem. Biodivers. 2021, 18, e2100085. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Liang, Z.C.; Chia, Y.C.; Lien, J.L.; Chen, K.S.; Lee, M.Y.; Wang, J.C. Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. J. Agric. Food Chem. 2006, 54, 2103–2110. [Google Scholar] [CrossRef]
- Milovanovic, I.; Zengin, G.; Maksimovic, S.; Tadic, V. Supercritical and ultrasound-assisted extracts from Pleurotus pulmonarius mushroom: Chemical profiles, antioxidative, and enzyme-inhibitory properties. J. Sci. Food Agric. 2021, 101, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- Abidin, M.H.; Abdullah, N.; Abidin, N.Z. Protective Effect of Antioxidant Extracts from Grey Oyster Mushroom, Pleurotus pulmonarius (Agaricomycetes), Against Human Low-Density Lipoprotein Oxidation and Aortic Endothelial Cell Damage. Int. J. Med. Mushrooms 2016, 18, 109–121. [Google Scholar] [CrossRef]
- Kim, J.H.; Sim, H.A.; Jung, D.Y.; Lim, E.Y.; Kim, Y.T.; Kim, B.J.; Jung, M.H. Poria cocus Wolf Extract Ameliorates Hepatic Steatosis through Regulation of Lipid Metabolism, Inhibition of ER Stress, and Activation of Autophagy via AMPK Activation. Int. J. Mol. Sci. 2019, 20, 4801. [Google Scholar] [CrossRef] [Green Version]
- Tel-Çayan, G.; Muhammad, A.; Duru, M.E.; Öztürk, M.; Adhikari, A.; Türkoğlu, A. A new fatty acid ester from an edible mushroom Rhizopogon luteolus. Nat. Prod. Res. 2016, 30, 2258–2264. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Muszyńska, B.; Szewczyk, A. Antioxidant components of selected indigenous edible mushrooms of the obsolete order Aphyllophorales. Rev. Iberoam. Micol. 2015, 32, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Cheng, X.L.; Liu, R.; Ho, C.C.; Wei, F.; Yan, S.H.; Lin, R.C.; Zhang, Y.; Sun, W.J. Pharmacokinetics of ergosterol in rats using rapid resolution liquid chromatography-atmospheric pressure chemical ionization multi-stage tandem mass spectrometry and rapid resolution liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1945–1953. [Google Scholar] [CrossRef]
- Dong, Z.; Iqbal, S.; Zhao, Z. Preparation of ergosterol-loaded nanostructured lipid carriers for enhancing oral bioavailability and antidiabetic nephropathy effects. AAPS PharmSciTech 2020, 21, 64. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Firempong, C.K.; Wang, Y.W.; Xu, W.Q.; Wang, M.M.; Cao, X.; Zhu, Y.; Tong, S.S.; Yu, J.N.; Xu, X.M. Ergosterol-loaded poly(lactide-co-glycolide) nanoparticles with enhanced in vitro antitumor activity and oral bioavailability. Acta Pharmacol. Sin. 2016, 37, 834–844. [Google Scholar] [CrossRef]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S.; Fleurat-Lessard, P.; Cruz, R.G.; Lafarge, C.; Grangeteau, C.; Yahou, F.; Gerbeau-Pissot, P.; Abrahão Júnior, O.; Gervais, P.; Simon-Plas, F. Antioxidant Properties of Ergosterol and Its Role in Yeast Resistance to Oxidation. Antioxidants 2021, 10, 1024. [Google Scholar] [CrossRef]
- Yongxia, Z.; Jian, X.; Suyuan, H.; Aixin, N.; Lihong, Z. Isolation and characterization of ergosterol from Monascus anka for anti-lipid peroxidation properties. J. Mycol. Médicale 2020, 30, 101038. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, C.; Wang, T.; Zhang, P.; Liu, Z.; Lu, C. Ergosterol attenuates LPS-induced myocardial injury by modulating oxidative stress and apoptosis in rats. Cell. Physiol. Biochem. 2018, 48, 583–592. [Google Scholar] [CrossRef]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Huan, W.; Tianzhu, Z.; Yu, L.; Shumin, W. Effects of Ergosterol on COPD in Mice via JAK3/STAT3/NF-κB Pathway. Inflammation 2017, 40, 884–893. [Google Scholar] [CrossRef]
- Sun, X.; Feng, X.; Zheng, D.; Li, A.; Li, C.; Li, S.; Zhao, Z. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo. Clin. Sci. 2019, 133, 1523–1536. [Google Scholar] [CrossRef]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264. 7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Yoo, M.-S.; Shin, J.-S.; Choi, H.-E.; Cho, Y.-W.; Bang, M.-H.; Baek, N.-I.; Lee, K.-T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-κB and p38 mitogen-activated protein kinase in RAW264. 7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, F.; Shen, M.; Jia, S.; Xie, J. Phytosterols suppress phagocytosis and inhibit inflammatory mediators via ERK pathway on LPS-triggered inflammatory responses in RAW264. 7 macrophages and the correlation with their structure. Foods 2019, 8, 582. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xiao, C.; Xu, H.; Yang, S.; Chen, Z.; Wang, H.; Zheng, B.; Mao, B.; Wu, X. Anti-inflammatory effects of Ganoderma lucidum sterols via attenuation of the p38 MAPK and NF-κB pathways in LPS-induced RAW 264.7 macrophages. Food Chem. Toxicol. 2021, 150, 112073. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabia, M.W.; Mohamed, G.A.; Ibrahim, S.R.M.; Asfour, H.Z. Anti-inflammatory ergosterol derivatives from the endophytic fungus Fusarium chlamydosporum. Nat. Prod. Res. 2021, 35, 5011–5020. [Google Scholar] [CrossRef]
- Zheng, M.S.; Hwang, N.K.; Kim, D.H.; Moon, T.C.; Son, J.K.; Chang, H.W. Chemical constituents of Melandrium firmum Rohrbach and their anti-inflammatory activity. Arch. Pharmacal Res. 2008, 31, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-J.; Jang, A.; Jang, H.-J.; Yang, K.-S. Inhibition of nitric oxide production, iNOS and COX-2 expression of ergosterol derivatives from Phellinus pini. Nat. Prod. Sci. 2012, 18, 147–152. [Google Scholar]
- Nallathamby, N.; Guan-Serm, L.; Vidyadaran, S.; Malek, S.N.A.; Raman, J.; Sabaratnam, V. Ergosterol of Cordyceps militaris attenuates LPS induced inflammation in BV2 microglia cells. Nat. Prod. Commun. 2015, 10, 1934578X1501000623. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-Y.; Xu, L.-T.; Li, A.-X.; Wang, S.-M. Effects of ergosterol, isolated from Scleroderma polyrhizum Pers., on lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation 2015, 38, 1979–1985. [Google Scholar] [CrossRef]
- Ikarashi, N.; Hoshino, M.; Ono, T.; Toda, T.; Yazawa, Y.; Sugiyama, K. A mechanism by which ergosterol inhibits the promotion of bladder carcinogenesis in rats. Biomedicines 2020, 8, 180. [Google Scholar] [CrossRef]
- Park, H.; Lee, T.H.; Chang, F.; Kwon, H.J.; Kim, J.; Kim, H. Synthesis of ergosterol and 5, 6-dihydroergosterol glycosides and their inhibitory activities on lipopolysaccharide-induced nitric oxide production. Bull. Korean Chem. Soc. 2013, 34, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-F.; Hsieh, C.-H.; Lin, W.-Y. Proteomic response of LAP-activated RAW 264.7 macrophages to the anti-inflammatory property of fungal ergosterol. Food Chem. 2011, 126, 207–212. [Google Scholar] [CrossRef]
- Ano, Y.; Kutsukake, T.; Hoshi, A.; Yoshida, A.; Nakayama, H. Identification of a novel dehydroergosterol enhancing microglial anti-inflammatory activity in a dairy product fermented with Penicillium candidum. PLoS ONE 2015, 10, e0116598. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, S.; Zhu, C.; Gao, Q.; Bai, J.; Si, J.; Chen, Y. Ergosterol ameliorates renal inflammatory responses in mice model of diabetic nephropathy. Biomed. Pharmacother. 2020, 128, 110252. [Google Scholar] [CrossRef] [PubMed]
- Kushairi, N.; Tarmizi, N.A.K.A.; Phan, C.W.; Macreadie, I.; Sabaratnam, V.; Naidu, M.; David, P. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends Food Sci. Technol. 2020, 104, 153–162. [Google Scholar] [CrossRef]
- Erol, E.; Ali, Z.; Oztürk, M.; Khan, S.; Khan, I.A. Inhibition of iNOS induction and nf-κΒ activation by taste compounds from the edible mushroom Tricholoma caligatum (Viv.) ricken. Rec. Nat. Prod. 2020, 14, 77–82. [Google Scholar] [CrossRef]
- Wu, S.-J.; Lu, T.-M.; Lai, M.-N.; Ng, L.-T. Immunomodulatory activities of medicinal mushroom Grifola frondosa extract and its bioactive constituent. Am. J. Chin. Med. 2013, 41, 131–144. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, H.J.; Lee, G.H.; Kim, D.S.; Kim, H.L.; Park, J.; Jung, Y.; Youn, D.H.; Kang, J.; Hong, S.H. Beneficial effects of the traditional medicine Igongsan and its constituent ergosterol on dextran sulfate sodium-induced colitis in mice. Mol. Med. Rep. 2015, 12, 3549–3556. [Google Scholar] [CrossRef] [Green Version]
- Durmus, A.; Durmus, I.; Bender, O.; Karatepe, O. The effect of Hericium erinaceum on the prevention of chemically induced experimental colitis in rats. Korean J. Intern Med. 2021, 36, S44–S52. [Google Scholar] [CrossRef]
- Kageyama-Yahara, N.; Wang, P.; Wang, X.; Yamamoto, T.; Kadowaki, M. The inhibitory effect of ergosterol, a bioactive constituent of a traditional Japanese herbal medicine saireito on the activity of mucosal-type mast cells. Biol. Pharm. Bull. 2010, 33, 142–145. [Google Scholar] [CrossRef] [Green Version]
- Kawai, J.; Andoh, T.; Mori, K. Suppression of leukotriene B4 production is involved in the anti-pruritic action of Grifola frondosa in pollen allergy-induced ocular itching in mice. Food Agric. Immunol. 2021, 32, 310–320. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospecting 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.A. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty. BioMed Res. Int. 2020, 2020, 8602879. [Google Scholar] [CrossRef]
- Chen, S.; Yong, T.; Zhang, Y.; Su, J.; Jiao, C.; Xie, Y. Anti-tumor and Anti-angiogenic Ergosterols from Ganoderma lucidum. Front. Chem. 2017, 5, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.Y.; Yang, F.L.; Li, L.H.; Rao, Y.K.; Ju, T.C.; Wong, W.T.; Hsieh, C.Y.; Pivkin, M.V.; Hua, K.F.; Wu, S.H. Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Sci. Rep. 2018, 8, 17956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sana, T.; Siddiqui, B.S.; Shahzad, S.; Farooq, A.D.; Siddiqui, F.; Sattar, S.; Begum, S. Antiproliferative Activity and Characterization of Metabolites of Aspergillus nidulans: An Endophytic Fungus from Nyctanthes arbor-tristis Linn. Against Three Human Cancer Cell Lines. Med. Chem. 2019, 15, 352–359. [Google Scholar] [CrossRef]
- Subbiah, M.T.; Abplanalp, W. Ergosterol (major sterol of baker’s and brewer’s yeast extracts) inhibits the growth of human breast cancer cells in vitro and the potential role of its oxidation products. Int. J. Vitam. Nutr. Res. 2003, 73, 19–23. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, X.; Yu, W.; Wang, R.; Xue, Z.; Kou, X. Identification and Evaluation of Bioactivity of Compounds from the Mushroom Pleurotus nebrodensis (Agaricomycetes) against Breast Cancer. Int. J. Med. Mushrooms 2017, 19, 839–848. [Google Scholar] [CrossRef]
- Pluchino, L.A.; Liu, A.K.; Wang, H.C. Reactive oxygen species-mediated breast cell carcinogenesis enhanced by multiple carcinogens and intervened by dietary ergosterol and mimosine. Free Radic. Biol. Med. 2015, 80, 12–26. [Google Scholar] [CrossRef]
- Chen, L.Y.; Sheu, M.T.; Liu, D.Z.; Liao, C.K.; Ho, H.O.; Kao, W.Y.; Ho, Y.S.; Lee, W.S.; Su, C.H. Pretreatment with an ethanolic extract of Taiwanofungus camphoratus (Antrodia camphorata) enhances the cytotoxic effects of amphotericin B. J. Agric. Food Chem. 2011, 59, 11255–11263. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lee, B.H.; Alagie, J.; Su, C.H. Combination treatment of ergosterol followed by amphotericin B induces necrotic cell death in human hepatocellular carcinoma cells. Oncotarget 2017, 8, 72727–72738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, Q.; Xie, Y.; Ding, Y.; Du, W.W.; Sdiri, M.; Yang, B.B. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget 2015, 6, 17832–17846. [Google Scholar] [CrossRef] [Green Version]
- Yazawa, Y.; Ikarashi, N.; Hoshino, M.; Kikkawa, H.; Sakuma, F.; Sugiyama, K. Inhibitory effect of ergosterol on bladder carcinogenesis is due to androgen signaling inhibition by brassicasterol, a metabolite of ergosterol. J. Nat. Med. 2020, 74, 680–688. [Google Scholar] [CrossRef]
- Muñoz-Fonseca, M.B.; Vidal-Limon, A.; Fernández-Pomares, C.; Rojas-Durán, F.; Hernández-Aguilar, M.E.; Espinoza, C.; Trigos, A.; Suárez-Medellín, J. Ergosterol exerts a differential effect on AR-dependent LNCaP and AR-independent DU-145 cancer cells. Nat. Prod. Res. 2021, 35, 4857–4860. [Google Scholar] [CrossRef] [PubMed]
- Takaku, T.; Kimura, Y.; Okuda, H. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. J. Nutr. 2001, 131, 1409–1413. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.; Pan, M.; Liu, H.; Tian, H.; Ye, Q.; Liu, H. Ergosterol peroxide inhibits ovarian cancer cell growth through multiple pathways. OncoTargets Ther. 2017, 10, 3467–3474. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Ye, M.; Zhou, Z. Aptamers: Novel diagnostic and therapeutic tools for diabetes mellitus and metabolic diseases. J. Mol. Med. 2017, 95, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Azushima, K.; Gurley, S.B.; Coffman, T.M. Modelling diabetic nephropathy in mice. Nat. Rev. Nephrol. 2018, 14, 48–56. [Google Scholar] [CrossRef]
- Xiong, M.; Huang, Y.; Liu, Y.; Huang, M.; Song, G.; Ming, Q.; Ma, X.; Yang, J.; Deng, S.; Wen, Y.; et al. Antidiabetic activity of ergosterol from Pleurotus ostreatus in KK-A(y) mice with spontaneous type 2 diabetes mellitus. Mol. Nutr. Food Res. 2018, 62, 1700444. [Google Scholar] [CrossRef]
- Ang, L.; Yuguang, L.; Liying, W.; Shuying, Z.; Liting, X.; Shumin, W. Ergosterol alleviates kidney injury in streptozotocin-induced diabetic mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 691594. [Google Scholar] [CrossRef] [Green Version]
- Holman, G.; Kasuga, M. From receptor to transporter: Insulin signalling to glucose transport. Diabetologia 1997, 40, 991–1003. [Google Scholar] [CrossRef] [Green Version]
- Vareda, P.M.P.; Saldanha, L.L.; Camaforte, N.A.d.P.; Violato, N.M.; Dokkedal, A.L.; Bosqueiro, J.R. Myrcia bella leaf extract presents hypoglycemic activity via PI3k/Akt insulin signaling pathway. Evid.-Based Complement. Altern. Med. 2014, 2014, 543606. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Sun, Y.; Wei, G.; Li, S.; Zhao, Z. Ergosterol ameliorates diabetic nephropathy by attenuating mesangial cell proliferation and extracellular matrix deposition via the TGF-β1/Smad2 signaling pathway. Nutrients 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Pardillo-Díaz, R.; Pérez-García, P.; Castro, C.; Nunez-Abades, P.; Carrascal, L. Oxidative Stress as a Potential Mechanism Underlying Membrane Hyperexcitability in Neurodegenerative Diseases. Antioxidants 2022, 11, 1511. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Chuchawankul, S.; Nilkhet, S.; Moungkote, N.; Sarachana, T.; Ung, A.T.; Baek, S.J.; Tencomnao, T. Ergosterol isolated from cloud ear mushroom (Auricularia polytricha) attenuates bisphenol A-induced BV2 microglial cell inflammation. Food Res. Int. 2022, 157, 111433. [Google Scholar] [CrossRef]
- Sillapachaiyaporn, C.; Mongkolpobsin, K.; Chuchawankul, S.; Tencomnao, T.; Baek, S.J. Neuroprotective effects of ergosterol against TNF-α-induced HT-22 hippocampal cell injury. Biomed. Pharmacother. 2022, 154, 113596. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Mbambo, B.; Odhav, B.; Mohanlall, V.J.J.M.P.R. Antifungal activity of stigmasterol, sitosterol and ergosterol from Bulbine natalensis Baker (Asphodelaceae). J. Med. Plants Res. 2012, 6, 5135–5141. [Google Scholar]
- Li, Y.; Song, Y.C.; Liu, J.Y.; Ma, Y.M.; Tan, R.X. Anti-Helicobacter pylori substances from endophytic fungal cultures. World J. Microbiol. Biotechnol. 2005, 21, 553–558. [Google Scholar] [CrossRef]
- Alexandre, T.R.; Lima, M.L.; Galuppo, M.K.; Mesquita, J.T.; do Nascimento, M.A.; dos Santos, A.L.; Sartorelli, P.; Pimenta, D.C.; Tempone, A.G. Ergosterol isolated from the basidiomycete Pleurotus salmoneostramineus affects Trypanosoma cruzi plasma membrane and mitochondria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, J.C.; Morais-Braga, M.F.; Guedes, G.M.; Tintino, S.R.; Freitas, M.A.; Menezes, I.R.; Coutinho, H.D. Enhancement of the antibiotic activity of aminoglycosides by alpha-tocopherol and other cholesterol derivates. Biomed. Pharmacother. Biomed. Pharmacother. 2014, 68, 1065–1069. [Google Scholar] [CrossRef]
- Andrade, J.C.; Morais Braga, M.F.B.; Guedes, G.M.M.; Tintino, S.R.; Freitas, M.A.; Quintans, L.J., Jr.; Menezes, I.R.A.; Coutinho, H.D.M. Cholecalciferol, Ergosterol, and Cholesterol Enhance the Antibiotic Activity of Drugs. Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam.—Ernahrungsforschung. J. Int. Vitaminol. Nutr. 2018, 88, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Tintino, S.R.; Oliveira-Tintino, C.D.; Campina, F.F.; Costa, M.S.; Cruz, R.P.; Pereira, R.L.; Andrade, J.C.; Sousa, E.O.; Siqueira-Junior, J.P.; Coutinho, H.D.; et al. Cholesterol and ergosterol affect the activity of Staphylococcus aureus antibiotic efflux pumps. Microb. Pathog. 2017, 104, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Fediuc, S.; Gaidhu, M.P.; Ceddia, R.B. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. J. Lipid Res. 2006, 47, 412–420. [Google Scholar] [CrossRef] [Green Version]

| Family | Species | Common Name | Reference |
|---|---|---|---|
| Agaricaceae | Agaricus bisporus | White button mushroom | [17] |
| Agaricus blazei | Sun mushroom | [18] | |
| Auriculariaceae | Auricularia auricula-judae | Wood ear mushroom | [17] |
| Auricularia polytricha | Wood ear mushroom | [19,20] | |
| Lycoperdacea | Calvatia excipuliformis | Pestle puffball | [21] |
| Agaricaceae | Coprinus comatus | Shaggy inkcap | [17] |
| Ganodermataceae | Amauroderma rugosum | Blood linzhi | [22] |
| Ganoderma lucidum | Lingzhi, Reishi | [17,23] | |
| Meripilaceae | Grifola frondosa | Maitake | [24,25] |
| Hericiaceae | Hericium erinaceus | Bearded tooth mushroom | [17] |
| Hericium novae-zealandiae | Pekepekekiore | [26] | |
| Hymenochaetaceae | Inonotus obliquus | Chaga mushroom | [27] |
| Russulaceae | Lactarius deliciosus | Saffron milk cap | [28] |
| Lactarius sanguifluus | Bloody milk cap | [28] | |
| Lactarius semisanguifluus | Semi-bloody milk cap | [28] | |
| Russula delica | Milk-white brittlegill mushroom | [28] | |
| Hydnangiaceae | Laccaria amethystina | Amethyst deceiver mushroom | [21] |
| Laccaria laccata | Deceiver mushroom | [21] | |
| Polyporaceae | Laetiporus sulphureus | Chicken of the woods | [21] |
| Boletaceae | Leccinum scabrum | Brown birch bolete | [21] |
| Agaricaceae | Lycoperdon perlatum | Gem-studded puffball | [21] |
| Marasmiaceae | Lentinula edodes | Shiitake | [17,29,30] |
| Agaricaceae | Macrolepiota procera | Parasol mushroom | [21] |
| Marasmiaceae | Marasmius oreades | Fairy ring champignon | [21] |
| Polyporaceae | Neolentinus lepideus | Scaly sawgill | [31] |
| Pleurotaceae | Pleurotus citrinopileatus | Golden oyster mushroom | [32] |
| Pleurotus eryngii | King oyster mushroom | [17] | |
| Pleurotus ostreatus | Oyster mushroom | [17] | |
| Pleurotus pulmonarius | Indian Oyster mushroom | [33,34] | |
| Polyporaceae | Poria cocos Wolf | Fu-ling, Indian bread | [35] |
| Rhizopogonaceae | Rhizopogon luteolus | Yellow false truffle | [36] |
| Sparassidaceae | Sparassis crispa | Cauliflower fungus | [37] |
| Suillaceae | Suillus bellinii | Champagne bolete | [28] |
| Suillus variegatus | Velvet bolete | [21] | |
| Boletaceae | Xerocomus badius | Bay bolete | [21] |
| Subject/Model | Dose | Outcome and Mechanism | Reference |
|---|---|---|---|
| tert-Butyl hydroperoxide-induced Saccharomyces cerevisiae | 0.83 mM | Lipid peroxidation $ | [44] |
| In Vitro non-cell-based assay | 11 μM | DPPH radical-scavenging activity # | [44] |
| Computational analysis (Gaussian 16 program) | N/A | Electron transfer followed by proton transfer mechanism # | [44] |
| In Vitro non-cell-based assays | 2 μg/mL | Lipid peroxidation $ | [45] |
| H2O2-induced primary dermal fibroblast (PCS-201-012) | 200 and 400 μg/mL | Intracellular ROS accumulation $ | [45] |
| LPS-induced Sprague Dawley rats | 25 and 50 mg/kg | Nrf2/HO-1 signaling # SOD level and activity # MDA level $ | [46] |
| LPS-treated H9C2 myoblast cells | 5–20 μM | Nrf2/HO-1 signaling # SOD activity # MDA level $ | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods 2023, 12, 2529. https://doi.org/10.3390/foods12132529
Rangsinth P, Sharika R, Pattarachotanant N, Duangjan C, Wongwan C, Sillapachaiyaporn C, Nilkhet S, Wongsirojkul N, Prasansuklab A, Tencomnao T, et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods. 2023; 12(13):2529. https://doi.org/10.3390/foods12132529
Chicago/Turabian StyleRangsinth, Panthakarn, Rajasekharan Sharika, Nattaporn Pattarachotanant, Chatrawee Duangjan, Chamaiphron Wongwan, Chanin Sillapachaiyaporn, Sunita Nilkhet, Nichaporn Wongsirojkul, Anchalee Prasansuklab, Tewin Tencomnao, and et al. 2023. "Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms" Foods 12, no. 13: 2529. https://doi.org/10.3390/foods12132529
APA StyleRangsinth, P., Sharika, R., Pattarachotanant, N., Duangjan, C., Wongwan, C., Sillapachaiyaporn, C., Nilkhet, S., Wongsirojkul, N., Prasansuklab, A., Tencomnao, T., Leung, G. P. -H., & Chuchawankul, S. (2023). Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods, 12(13), 2529. https://doi.org/10.3390/foods12132529