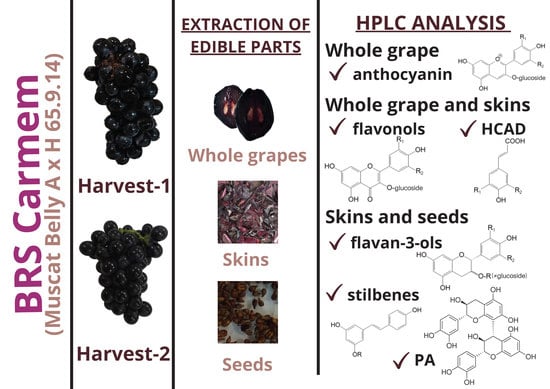
Phenolic Composition of Brazilian BRS Carmem (Muscat Belly A × BRS Rúbea) Grapes: Evaluation of Their Potential Use as Bioingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Grapes
2.3. Physicochemical Characteristics
2.4. HPLC-DAD-ESI-MS2 Identification and Quantification of Phenolic Compounds of Grape Parts
2.5. Data Analysis
3. Results
3.1. Physicochemical Characteristics
3.2. Qualitative and Quantitative Determination of PC by HPLC-DAD-ESI-MS2
3.2.1. Flavonols and Anthocyanins
3.2.2. Qualitative and Quantitative Profiles of Stilbenes, Flavan-3-ol Monomers and Dimers and Proanthocyanidins
3.2.3. Qualitative and Quantitative Profiles of Hydroxycinnamic Acid Derivatives and Stilbenes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). Fruit and Vegetables–Your Dietary Essentials: The International Year of Fruits and Vegetables, 2021, Background Paper; FAO: Rome, Italy, 2020. [Google Scholar]
- Dos Santos, K.L.; Panizzon, J.; Cenci, M.M.; Grabowski, G.; Jahno, V.D. Perdas e desperdícios de alimentos: Reflexões sobre o atual cenário brasileiro. Braz. J. Food Technol. 2020, 23, e2019134. [Google Scholar] [CrossRef]
- Awan, U.A.; Saqib, M.A.N.; Ahmed, H.; Afzal, M.S. Hunger, malnutrition and persistent COVID-19 lockdowns: A swift approach is required. J. Formos. Med. Assoc. 2021, 120, 1539–1540. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Rampal, J. The COVID-19 pandemic and food insecurity: A viewpoint on India. World Dev. 2020, 135, 105068. [Google Scholar] [CrossRef]
- Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Natural blue food colorants: Consumer acceptance, current alternatives, trends, challenges, and future strategies. Trends Food Sci. Technol. 2021, 112, 163–173. [Google Scholar] [CrossRef]
- Blancquaert, E.; Oberholster, A.; Ricardo-Da-Silva, J.M.; Deloire, A. Effects of Abiotic Factors on Phenolic Compounds in the Grape Berry—A Review. South Afr. J. Enol. Vitic. 2018, 40, 1. [Google Scholar] [CrossRef]
- Ahmed, S.; Roberto, S.R.; Shahab, M.; Colombo, R.C.; Silvestre, J.P.; Koyama, R.; de Souza, R.T. Proposal of double-cropping system for ‘BRS Isis’ seedless grape grown in subtropical area. Sci. Hortic. 2019, 251, 118–126. [Google Scholar] [CrossRef]
- Olivati, C.; Nishiyama, Y.P.; da Silva, R.; Gómez-Alonso, S.; Lago-Vanzela, E.S. BRS Clara raisins production: Effect of the pre-treatment and the drying process on the phenolic composition. J. Food Compos. Anal. 2022, 114, 104771. [Google Scholar] [CrossRef]
- Leng, F.; Zhou, J.; Wang, C.; Sun, L.; Zhang, Y.; Li, Y.; Wang, L.; Wang, S.; Zhang, X.; Xie, Z. Post-veraison different frequencies of water deficit strategies enhance Reliance grapes quality under root restriction. Food Chem. 2022, 390, 133181. [Google Scholar] [CrossRef]
- Oliveira, A.; Amaro, A.L.; Pintado, M. Impact of food matrix components on nutritional and functional properties of fruit-based products. Curr. Opin. Food Sci. 2018, 22, 153–159. [Google Scholar] [CrossRef]
- Da Silva, M.J.R.; Paiva, A.P.M.; Pimentel, A.; Sánchez, C.A.P.C.; Callili, D.; Moura, M.; Leonel, S.; Tecchio, M.A. Yield performance of new juice grape varieties grafted onto different rootstocks under tropical conditions. Sci. Hortic. 2018, 241, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, W.-K.; Gao, X.-T.; He, L.; Yang, X.-H.; He, F.; Duan, C.-Q.; Wang, J. Rootstock-Mediated Effects on Cabernet Sauvignon Performance: Vine Growth, Berry Ripening, Flavonoids, and Aromatic Profiles. Int. J. Mol. Sci. 2019, 20, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao-Takai, M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. A low temperature promotes anthocyanin biosynthesis but does not accelerate endogenous abscisic acid accumulation in red-skinned grapes. Plant Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.; Roberto, S.R.; Ahmed, S.; Colombo, R.C.; Silvestre, J.P.; Koyama, R.; de Souza, R.T. Relationship between anthocyanins and skin color of table grapes treated with abscisic acid at different stages of berry ripening. Sci. Hortic. 2019, 259, 108859. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Herrera-Bustamante, B.; López-Solís, R. Ripening-associated flattening out of inter-varietal differences in some groups of phenolic compounds in the skins of six emblematic grape wine varieties. J. Food Compos. Anal. 2021, 99, 103858. [Google Scholar] [CrossRef]
- Tecchio, M.A.; da Silva, M.J.R.; Cunha, S.R.; Callili, D.; Sánchez, C.A.P.C.; de Souza, J.R.; Moura, M.F. Productive performance and physicochemical quality of grapes for processing grown on different rootstocks. Pesqui. Agropecuária Bras. 2022, 57, s1678–s3921. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef]
- Xie, L.; Su, H.; Sun, C.; Zheng, X.; Chen, W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2017, 72, 13–24. [Google Scholar] [CrossRef]
- Da Silva, M.J.R.; Padilha, C.V.D.S.; Lima, M.D.S.; Pereira, G.E.; Filho, W.G.V.; Moura, M.F.; Tecchio, M.A. Grape juices produced from new hybrid varieties grown on Brazilian rootstocks—Bioactive compounds, organic acids and antioxidant capacity. Food Chem. 2019, 289, 714–722. [Google Scholar] [CrossRef]
- De Castilhos, M.B.M.; Corrêa, O.L.D.S.; Zanus, M.C.; Maia, J.D.G.; Gómez-Alonso, S.; García-Romero, E.; Del Bianchi, V.L.; Hermosín-Gutiérrez, I. Pre-drying and submerged cap winemaking: Effects on polyphenolic compounds and sensory descriptors. Part II: BRS Carmem and Bordô (Vitis labrusca L.). Food Res. Int. 2015, 76, 697–708. [Google Scholar] [CrossRef] [Green Version]
- De Castilhos, M.B.M.; Del Bianchi, V.L.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Sensory descriptive and comprehensive GC–MS as suitable tools to characterize the effects of alternative winemaking procedures on wine aroma. Part I: BRS Carmem and BRS Violeta. Food Chem. 2018, 272, 462–470. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Gómez, M.V.; Velders, A.H.; Hermosín-Gutiérrez, I. Flavonol 3-O-Glycosides Series of Vitis vinifera Cv. Petit Verdot Red Wine Grapes. J. Agric. Food Chem. 2008, 57, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Rebello, L.P.G.; Lago-Vanzela, E.S.; Barcia, M.T.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Phenolic composition of the berry parts of hybrid grape cultivar BRS Violeta (BRS Rubea×IAC 1398-21) using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2013, 54, 354–366. [Google Scholar] [CrossRef]
- World Geodetic System. World Geodetic System-1984 (WGS-84) Manual, 2nd ed.; International Civil Aviation Organization: Montreal, QC, Canada, 1984. [Google Scholar]
- Cepagri. Centro de Pesquisas Meteorológicas e Climáticas Aplicadas à Agricultura. Clima dos Municípios Paulistas. Available online: https://www.cpa.unicamp.br/ (accessed on 18 January 2017).
- Santos, H.G.D.; Jacomine, P.K.T.; Anjos, L.H.C.D.; Oliveira, V.A.D.; Lumbreras, J.F.; Coelho, M.R.; De-Almeida, J.A.; De-Araújo Filho, J.C.; De-Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; p. 356. [Google Scholar]
- USDA-Natural Resources Conservation Service. Soil Survey Staff, Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 360.
- AOAC-Association of Official Analytical Chemists International. Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.; AOAC-Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Colombo, R.C.; Roberto, S.R.; Nixdorf, S.L.; Pérez-Navarro, J.; Gómez-Alonso, S.; Mena-Morales, A.; García-Romero, E.; Gonçalves, L.S.A.; da Cruz, M.A.; de Carvalho, D.U.; et al. Analysis of the phenolic composition and yield of ‘BRS Vitoria’ seedless table grape under different bunch densities using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2020, 130, 108955. [Google Scholar] [CrossRef] [PubMed]
- Tavares, I.M.D.C.; de Castilhos, M.B.M.; Mauro, M.A.; Ramos, A.M.; de Souza, R.T.; Gómez-Alonso, S.; Gomes, E.; Da-Silva, R.; Hermosín-Gutiérrez, I.; Lago-Vanzela, E.S. BRS Violeta (BRS Rúbea × IAC 1398-21) grape juice powder produced by foam mat drying. Part I: Effect of drying temperature on phenolic compounds and antioxidant activity. Food Chem. 2019, 298, 124971. [Google Scholar] [CrossRef] [PubMed]
- Camargo, U.A.; Maia, J.D.G.; Ritschel, P.S. BRS Carmem-Nova Cultivar de uva Tardia Para Suco, Comunicado Técnico 84. 2008. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPUV/9543/1/cot084.pdf (accessed on 28 February 2017).
- Nishiyama, Y.P.O. Composição Fenólica de Uvas Brasileira e Produtos Derivados/Composición Fenólica de uvas Brasileñas y Productos Derivados. Ph.D. Thesis, Univeside Estaudal Paulista/Universidad de Castilla-la Mancha, São José do Rio Preto, Brazil, 14 July 2020. [Google Scholar]
- Cheng, J.; Wei, L.; Mei, J.; Wu, J. Effect of rootstock on phenolic compounds and antioxidant properties in berries of grape (Vitis vinifera L.) cv. ‘Red Alexandria’. Sci. Hortic. 2017, 217, 137–144. [Google Scholar] [CrossRef]
- Borges, R.S.; Roberto, S.R.; Yamashita, F.; Assis, A.M.; Yamamoto, L.Y. Produção e Qualidade de Frutos de Clones de Videira ‘Concord’ Sobre Diferentes Porta-Enxertos. Pesqui. Agropecuária Trop. 2014, 44, 198–204. Available online: https://www.scielo.br/j/pat/a/mscdwMHWbbsyZqQCdR79mKD/?format=pdf&lang=pt (accessed on 28 February 2017).
- De Brito, A.L.; Bonfim, W.M.D.; Neto, E.R.D.A.; de Lima, M.A.C. Quality and antioxidant potential of ‘BRS clara’ and ‘Arizul’ grapes influenced by rootstocks in a tropical region. Ciên. Agrotecnol. 2019, 43. [Google Scholar] [CrossRef] [Green Version]
- Ritschel, P.S.; Maia, J.D.G.; Camargo, U.A.; Zanus, M.C.; Souza, R.T.; Fajardo, T.V.M. ‘BRS Magna’ nova cultivar de uva para suco com ampla adaptação climática, Bento Gonçalves: EMBRAPA. Comun. Técnico 2012, 125. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/71803/1/cot125.pdf (accessed on 28 February 2017).
- Camargo, U.A.; Maia, J.D.G.; Nachtigal, J.C. BRS Violeta: Nova cultivar de uva para suco e vinho de mesa, Bento Gonçalves: EMBRAPA Comun. Técnico. Comun. Técnico 2005, 63. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPUV/7590/1/cot063.pdf (accessed on 28 February 2017).
- De Assis, A.M.; Yamamoto, L.Y.; de Souza, F.S.; Borges, R.d.S.; Roberto, S.R. Evolução da maturação e características físico-químicas e produtivas das videiras ‘BRS Carmem’ e ‘Isabel’. Rev. Bras. Frutic. 2011, 33, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, L.Y.; de Assis, A.M.; Morais, H.; de Souza, F.S.; Miotto, L.C.V.; Sato, A.J.; de Souza, R.T.; Roberto, S.R. Evolução da maturação da uva ‘BRS Clara’ sob cultivo protegido durante a safra fora de época. Bragantia 2011, 70, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Da Mota, R.V.; Silva, C.P.C.; Favero, A.C.; Purgatto, E.; Shiga, T.; Regina, M.D.A. Composição físico-química de uvas para vinho fino em ciclos de verão e inverno. Rev. Bras. Frutic. 2010, 32, 1127–1137. [Google Scholar] [CrossRef] [Green Version]
- Junior, W.J.F.L.; Nadai, C.; Crepalde, L.T.; de Oliveira, V.; de Matos, A.D.; Giacomini, A.; Corich, V. Potential use of Starmerella bacillaris as fermentation starter for the production of low-alcohol beverages obtained from unripe grapes. Int. J. Food Microbiol. 2019, 303, 1–8. [Google Scholar] [CrossRef]
- De Matos, A.D.; Curioni, A.; Bakalinsky, A.T.; Marangon, M.; Pasini, G.; Vincenzi, S. Chemical and sensory analysis of verjuice: An acidic food ingredient obtained from unripe grape berries. Innov. Food Sci. Emerg. Technol. 2017, 44, 9–14. [Google Scholar] [CrossRef]
- Fia, G.; Gori, C.; Bucalossi, G.; Borghini, F.; Zanoni, B. A Naturally Occurring Antioxidant Complex from Unripe Grapes: The Case of Sangiovese (v. Vitis vinifera). Antioxidants 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucalossi, G.; Fia, G.; Dinnella, C.; De Toffoli, A.; Canuti, V.; Zanoni, B.; Servili, M.; Pagliarini, E.; Toschi, T.G.; Monteleone, E. Functional and sensory properties of phenolic compounds from unripe grapes in vegetable food prototypes. Food Chem. 2020, 315, 126291. [Google Scholar] [CrossRef] [PubMed]
- Belviso, S.; Torchio, F.; Novello, V.; Giacosa, S.; de Palma, L.; Segade, S.R.; Gerbi, V.; Rolle, L. Modeling of the evolution of phenolic compounds in berries of “Italia” table grape cultivar using response surface methodology. J. Food Compos. Anal. 2017, 62, 14–22. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; Da-Silva, R.; Gomes, E.; García-Romero, E.; Hermosín-Gutiérrez, I. Phenolic Composition of the Edible Parts (Flesh and Skin) of Bordô Grape (Vitis labrusca) Using HPLC–DAD–ESI-MS/MS. J. Agric. Food Chem. 2011, 59, 13136–13146. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Martín-Baz, A.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Anthocyanin and flavonol profiles of Vitis vinifera L. cv Rufete grapes. Biochem. Syst. Ecol. 2014, 53, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Favre, G.; González-Neves, G.; Piccardo, D.; Gómez-Alonso, S.; Pérez-Navarro, J.; Hermosín-Gutiérrez, I. New acylated flavonols identified in Vitis vinifera grapes and wines. Food Res. Int. 2018, 112, 98–107. [Google Scholar] [CrossRef]
- Liang, N.-N.; Zhu, B.-Q.; Han, S.; Wang, J.-H.; Pan, Q.-H.; Reeves, M.J.; Duan, C.-Q.; He, F. Regional characteristics of anthocyanin and flavonol compounds from grapes of four Vitis vinifera varieties in five wine regions of China. Food Res. Int. 2014, 64, 264–274. [Google Scholar] [CrossRef]
- Fraige, K.; Pereira-Filho, E.R.; Carrilho, E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC–DAD–MS and exploratory analysis by principal component analysis. Food Chem. 2014, 145, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.C.; Roberto, S.R.; da Cruz, M.A.; de Carvalho, D.U.; Yamamoto, L.Y.; Nixdorf, S.L.; Pérez-Navarro, J.; Gómez-Alonso, S.; Shahab, M.; Ahmed, S.; et al. Characterization of the phenolic ripening development of ‘BRS Vitoria’ seedless table grapes using HPLC–DAD–ESI-MS/MS. J. Food Compos. Anal. 2021, 95, 103693. [Google Scholar] [CrossRef]
- De Oliveira, J.B.; Egipto, R.; Laureano, O.; de Castro, R.; Pereira, G.E.; Ricardo-Da-Silva, J.M. Climate effects on physicochemical composition of Syrah grapes at low and high altitude sites from tropical grown regions of Brazil. Food Res. Int. 2019, 121, 870–879. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, K.; Roberto, S.R.; Chiarotti, F.; Koyama, R.; Hussain, I.; de Souza, R.T. Control of Botrytis mold of the new seedless grape ‘BRS Vitoria’ during cold storage. Sci. Hortic. 2015, 193, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Tsimplouli, C.; Demetzos, C.; Hadzopoulou-Cladaras, M.; Pantazis, P.; Dimas, K. In vitro activity of dietary flavonol congeners against human cancer cell lines. Eur. J. Nutr. 2011, 51, 181–190. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Asp. Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of Microencapsulation by Spray Drying and Freeze Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.C.; de Souza, V.B.; Thomazini, M.; da Silva, E.R.; Smaniotto, T.; de Carvalho, R.A.; Genovese, M.I.; Favaro-Trindade, C.S. Use of the jabuticaba (Myrciaria cauliflora) depulping residue to produce a natural pigment powder with functional properties. LWT 2014, 55, 203–209. [Google Scholar] [CrossRef]
- Kuck, L.S.; Wesolowski, J.L.; Noreña, C.P.Z. Effect of temperature and relative humidity on stability following simulated gastro-intestinal digestion of microcapsules of Bordo grape skin phenolic extract produced with different carrier agents. Food Chem. 2017, 230, 257–264. [Google Scholar] [CrossRef]
- Assous, M.; Abdel-Hady, M.; Medany, G.M. Evaluation of red pigment extracted from purple carrots and its utilization as antioxidant and natural food colorants. Ann. Agric. Sci. 2014, 59, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mohammadabadi, S.S.; Goli, M.; Tabasi, S.N. Optimization of Bioactive Compound Extraction from Eggplant Peel by Response Surface Methodology: Ultrasound-Assisted Solvent Qualitative and Quantitative Effect. Foods 2022, 11, 3263. [Google Scholar] [CrossRef]
- Gutiérrez, I.H.; Lorenzo, E.S.-P.; Espinosa, A.V. Phenolic composition and magnitude of copigmentation in young and shortly aged red wines made from the cultivars, Cabernet Sauvignon, Cencibel, and Syrah. Food Chem. 2005, 92, 269–283. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Darra, N.; El Kantar, S.; Hobaika, Z.; Louka, N.; Maroun, R.G. A Comparative Study of the Phenolic and Technological Maturities of Red Grapes Grown in Lebanon. Antioxidants 2017, 6, 8. [Google Scholar] [CrossRef]
- Yamamoto, L.Y.; de Assis, A.M.; Roberto, S.R.; Bovolenta, Y.R.; Nixdorf, S.L.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Application of abscisic acid (S-ABA) to cv. Isabel grapes (Vitis vinifera×Vitis labrusca) for color improvement: Effects on color, phenolic composition and antioxidant capacity of their grape juice. Food Res. Int. 2015, 77, 572–583. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Gómez-Alonso, S.; Godoy, H.T.; Hermosín-Gutiérrez, I. Phenolic composition of grape and winemaking by-products of Brazilian hybrid cultivars BRS Violeta and BRS Lorena. Food Chem. 2014, 159, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.R.; Rodrigues, A.A.M.; De Vasconcelos, V.A.F.; Costa, J.P.D.; De Lima, M.A.C. Trellis systems, rootstocks and season influence on the phenolic composition of ‘Chenin Blanc’ grape. Plant Physiol. Biochem. 2020, 77, 3. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Harvest 1 | Harvest 2 | |
|---|---|---|
| Moisture (%) | 74.4 ± 0.16 | 81.50 ± 0.46 |
| pH | 3.56 ± 0.29 | 3.75 ± 0.04 |
| TA (tartaric acid g•100 g−1) | 0.82 ± 0.10 | 1.16 ± 0.16 |
| SS (°Brix at 25 °C) | 19.50 ± 0.00 | 12.97 ± 0.06 |
| SS/TA ratio | 23.93 ± 2.82 | 11.34 ± 1.43 |
| L × W (mm × mm) | 15.5 × 13.6 | 17.6 × 15.6 |
| Berry mass (g) | 2.28 ± 0.31 | 2.36 ± 0.23 |
| Flavonol ¹ | % Molar Percentages | |||
|---|---|---|---|---|
| WG1 | SK1 | WG2 | SK2 | |
| M-glcU | 4.98 ± 0.55aB | 4.60 ± 0.67aB | 9.07 ± 0.34aA | 9.72 ± 0.61aA |
| M-gal | 2.39 ± 0.36a | 1.29 ± 0.01a | ND | ND |
| M-glc | 72.23 ± 0.62aA | 56.97 ± 6.78aA | 41.96 ± 0.31aB | 38.40 ± 3.79aA |
| Q-gal | ND | ND | 0.99 ± 0.08a | 0.68 ± 0.62a |
| Q-glcU | 5.55 ± 0.68aA | 4.70 ± 1.77aB | 10.68 ± 1.72aA | 15.05 ± 0.14aA |
| Q-glc | 4.84 ± 0.10aB | 3.93 ± 1.44aB | 10.63 ± 1.61aA | 11.71 ± 2.33aA |
| L-glc | 6.33 ± 0.46bB | 9.73 ± 0.99aB | 16.14 ± 2.10aA | 14.05 ± 0.19aA |
| I-glc | 0.10 ± 0.03aB | 0.1 0.88aB | 2.25 ± 0.07aA | 2.14 ± 0.35aA |
| S-gal | 0.42 ± 0.05a | 0.24 ± 0.05a | ND | ND |
| S-glc | 1.71 ± 0.18aB | 1.74 ± 0.54aB | 7.44 ± 1.33aA | 7.48 ± 0.60aA |
| S-cmglc | ND | ND | 0.83 ± 0.08a | 0.76 ± 0.17a |
| M free | 1.44 ± 0.12b | 12.63 ± 0.80a | ND | |
| Q free | ND | 1.78 ± 0.85 | ND | ND |
| L free | ND | 0.57 ± 0.20 | ND | ND |
| I free | ND | 0.43 ± 0.38 | ND | ND |
| S free | ND | 1.28 ± 0.73 | ND | ND |
| M-type | 81.04 ± 1.40aA | 75.49 ± 6.92aA | 51.03 ± 0.03aB | 48.12 ± 4.41aB |
| Q-type | 10.39 ± 0.78aB | 10.40 ± 4.06aB | 22.31 ± 3.41aA | 27.45 ± 3.09aA |
| L-type | 6.33 ± 0.46bB | 10.30 ± 1.19aB | 16.14 ± 2.10aA | 14.05 ± 0.19aA |
| I-type | 0.10 ± 0.03aB | 0.54 ± 0.46aA | 2.25 ± 0.07aA | 2.14 ± 0.35aA |
| S-type | 2.14 ± 0.13aB | 3.27 ± 1.21aB | 8.27 ± 0.41aA | 8.25 ± 0.78aA |
| Total (mg Q-3-glc/kg fruit) | 70.54 ± 11.11aA | 68.48 ± 4.28aA | 75.79 ± 8.00aA | 38.66 ± 3.83bB |
| Anthocyanin 1 | % Molar Porcentages 2 | |
|---|---|---|
| WG1 | WG2 | |
| dp-3,5-diglc | 25.11 ± 2.94A | 0.48 ± 0.15B |
| cy-3,5-diglc | 1.48 ± 0.09A | 0.05 ± 0.02B |
| pt-3,5-diglc | 13.11 ± 0.87A | 0.97 ± 0.28B |
| pn-3,5-diglc | 3.09 ± 0.75A | 1.73 ± 0.24A |
| mv-3,5-diglc | 14.10 ± 1.50A | 15.14 ± 2.31A |
| pt-3-acglc-5-glc | ND | 0.25 ± 0.04 |
| pn-3-acglc-5-glc | 0.18 ± 0.03B | 0.37 ± 0.05A |
| mv-3-acglc-5-glc | 0.78 ± 0.20B | 2.55 ± 0.17A |
| dp-3-cmglc-5-glc | 25.81 ± 1.59A | 6.98 ± 0.39B |
| cy-3-cmglc-5-glc | 1.84 ± 0.38A | 0.43 ± 0.12B |
| pt-3-cmglc-5-glc | 4.70 ± 0.48B | 13.03 ± 0.91A |
| pn-3-cmglc-5-glc | 1.62 ± 0.45B | 3.34 ± 0.25A |
| mv-3-cmglc-5-glc | 7.79 ± 1.70B | 52.49 ± 4.34A |
| dp-3-cfglc-5-glc | 0.38 ± 0.06 | ND |
| cy-3-cfglc-5-glc | ND | 0.01 ± 0.00 |
| pt-3-cfglc-5-glc | ND | 0.57 ± 0.04 |
| pn-3-cfglc-5-glc | ND | 0.42 ± 0.19 |
| mv-3-cfglc-5-glc | ND | 1.21 ± 0.08 |
| dp-3-glc | 30.14 ± 1.49A | 4.05 ± 1.25B |
| cy-3-glc | 3.86 ± 0.18A | 0.70 ± 0.14B |
| pt-3-glc | 6.67 ± 1.04A | 2.31 ± 0.24B |
| pn-3-glc | 1.94 ± 0.62A | 2.58 ± 0.27A |
| mv-3-glc | 3.91 ± 0.73A | 5.66 ± 0.92A |
| dp-3-acglc | 2.32 ± 0.41A | 1.85 ± 0.40A |
| pt-3-acglc | 1.40 ± 0.19A | 1.29 ± 0.02A |
| pn-3-acglc | ND | 2.74 ± 0.31 |
| mv-3-acglc | 0.94 ± 0.22B | 2.54 ± 0.27A |
| dp-3-cmglc | 39.83 ± 2.69A | 27.76 ± 0.02B |
| cy-3-cmglc | 1.92 ± 0.14 | ND |
| pt-3-cmglc | 4.44 ± 0.15B | 19.12 ± 0.90A |
| pn-3-cmglc | 0.64 ± 0.15B | 2.28 ± 0.25A |
| mv-3-cmglc | 1.98 ± 0.36B | 25.65 ± 2.25A |
| mv-3-cfglc | ND | 1.46 ± 0.22 |
| Ratio 3,5-diglc/3-glc | 2.22 ± 0.16B | 5.64 ± 0.23A |
| mg/kg fruit (mv-3,5-diglc) | 1650.19 ± 110.65A | 1631.41 ± 127.37A |
| mg/kg fruit (mv-3-glc) | 559.49 ± 2.92A | 194.51 ± 23.18B |
| Total mg/kg fruit (mv-3,5-diglc) | 2528.01 ± 106.08A | 1921.58 ± 161.95B |
| Compound 1 | mg/kg Fruit 2 | |||
|---|---|---|---|---|
| SE1 | SK1 | SE2 | SK2 | |
| C | 56.10 ± 0.79aB | 0.62 ± 0.11bA | 84.24 ± 1.21aA | 0.65 ± 0.04bA |
| EC | 18.66 ± 0.92aB | 0.15 ± 0.00bA | 40.50 ± 3.72aA | 0.11 ± 0.01bB |
| GC | 0.06 ± 0.00b | 0.19 ± 0.02aA | ND | 0.16 ± 0.04A |
| EGC | 0.21 ± 0.04a | 0.21 ± 0.01aA | ND | 0.12 ± 0.01B |
| CG | 0.09 ± 0.00B | ND | 0.13 ± 0.00A | ND |
| ECG | 0.81 ± 0.01aA | 0.02 ± 0.02bA | 1.15 ± 0.11aA | 0.04 ± 0.00bA |
| MG1 | ND | ND | 1.97 ± 0.35 | ND |
| MG2 | ND | ND | 0.55 ± 0.01 | ND |
| Total monomers 3 | 179.05 ± 18.29aA | 1.17 ± 0.15bA | 127.18 ± 5.08aB | 1.04 ± 0.07bA |
| PB1 | 7.04 ± 1.12aB | 0.08 ± 0.01bA | 17.59 ± 0.83aA | 2.93 ± 0.25bA |
| PB2 | 1.12 ± 0.06aB | 0.08 ± 0.01bB | 35.41 ± 0.15aA | 0.42 ± 0.08bA |
| PB4 | 12.23 ± 0.38aA | 0.18 ± 0.01bA | 2.40 ± 0.02aB | 0.16 ± 0.03bA |
| Total dimers 3 | 24.15 ± 0.20aB | 0.73 ± 0.01bB | 55.54 ± 0.71aA | 3.52 ± 0.35bA |
| Total 3 | 203.20 ± 18.49aA | 1.90 ± 0.16bB | 182.71 ± 4.37aA | 4.56 ± 0.36bA |
| PA 3 | 453.57 ± 79.99aA | 37.58 ± 0.04bB | 299.86 ± 10.82aA | 98.92 ± 9.18bA |
| mDP | 3.06 ± 0.19bA | 5.93 ± 0.21aA | 3.64 ± 0.36bA | 5.65 ± 0.07aA |
| % galloylation | 8.71 ± 0.86aA | 1.95 ± 0.12bB | 7.25 ± 0.29aA | 3.95 ± 0.07bA |
| % prodelphinidin | 0.81 ± 0.06bA | 25.02 ± 0.93aA | 0.46 ± 0.08bB | 16.91 ± 1.68aB |
| % C-term | 48.13 ± 7.33aA | 60.28 ± 1.14aA | 64.68 ± 1.78aA | 62.06 ± 2.62aA |
| % EC-term | 41.56 ± 5.58aA | 11.56 ± 0.24bA | 31.04 ± 1.55aA | 11.04 ± 1.36bA |
| % GC-term | 0.18 ± 0.12b | 13.40 ± 0.29aA | ND | 13.99 ± 2.58A |
| % EGC-term | 0.37 ± 0.04b | 13.21 ± 0.44aA | ND | 10.59 ± 0.79B |
| % CG-term | 0.04 ± 0.01A | ND | 0.06 ± 0.00A | ND |
| % ECG-term | 9.73 ± 1.81aA | 1.54 ± 0.17bB | 0.56 ± 0.08bB | 2.33 ± 0.02aA |
| % MG-term | ND | ND | 3.65 ± 0.30 | ND |
| % C-ext | 5.01 ± 5.00aA | 0.58 ± 0.1aB | 10.49 ± 0.80aA | 3.90 ± 0.36bA |
| % EC-ext | 86.95 ± 5.61aA | 73.98 ± 1.39bA | 78.87 ± 0.69aA | 70.77 ± 2.38bA |
| % GC-ext | 0.05 ± 0.00b | 0.47 ± 0.08a | ND | ND |
| % EGC-ext | 0.56 ± 0.04bA | 22.97 ± 1.22aA | 0.64 ± 0.09bA | 20.53 ± 2.02aA |
| % CG-ext | 7.43 ± 0.33aB | 1.99 ± 0.15bB | 10.00 ± 0.03aA | 4.80 ± 0.09bA |
| HCAD | % Molar Percentage | |||
|---|---|---|---|---|
| WG1 | SK1 | WG2 | SK2 | |
| trans-caftaric | 53.36 ± 2.015aA | 50.76 ± 3.41aA | 24.77 ± 5.31aB | 33.56 ± 4.55aB |
| trans-cutaric | 18.94 ± 2.00aA | 21.27 ± 5.46aA | 13.69 ± 5.60aA | 11.74 ± 0.90aB |
| 1-glc-cumaric | 14.61 ± 1.29aA | 6.96 ± 5.20aA | 16.95 ± 1.44aA | 13.63 ± 1.03bA |
| 2-glc-cumaric | 6.16 ± 0.60aB | 8.26 ± 2.06aA | 14.63 ± 1.16aA | 12.80 ± 4.46aA |
| trans-fertaric | 1.89 ± 0.33bB | 7.05 ± 0.48aA | 8.88 ± 0.83aA | 9.47 ± 4.70aA |
| 3-glc-cumaric | 5.04 ± 1.07aB | 5.70 ± 1.11aB | 21.08 ± 0.67aA | 18.80 ± 3.28aA |
| Total 1 | 383.98 ± 32.72aA | 173.95 ± 91.36bA | 67.09 ± 21.69aB | 21.74 ± 4.00bB |
| Stilbenes | % Molar Percentage | |||
|---|---|---|---|---|
| SE1 | SK1 | SE2 | SK2 | |
| trans-piceid | 98.73 ± 0.71aA | 78.79 ± 3.20bA | 1.94 ± 0.26bB | 29.88 ± 6.73aB |
| cis-piceid | 1.27 ± 0.71bB | 21.21 ± 3.20aA | 6.95 ± 0.36aA | 4.96 ± 1.51aA |
| cis-resveratrol | ND | ND | 91.11 ± 0.10a | 65.16 ± 8.06b |
| Total 1 | 48.67 ± 4.76aB | 21.87 ± 4.44bA | 896.25 ± 265.34aA | 3.69 ± 0.45bB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama-Hortense, Y.P.; Olivati, C.; Pérez-Navarro, J.; Souza, R.T.; Janzantti, N.S.; Da-Silva, R.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Lago-Vanzela, E.S. Phenolic Composition of Brazilian BRS Carmem (Muscat Belly A × BRS Rúbea) Grapes: Evaluation of Their Potential Use as Bioingredients. Foods 2023, 12, 2608. https://doi.org/10.3390/foods12132608
Nishiyama-Hortense YP, Olivati C, Pérez-Navarro J, Souza RT, Janzantti NS, Da-Silva R, Hermosín-Gutiérrez I, Gómez-Alonso S, Lago-Vanzela ES. Phenolic Composition of Brazilian BRS Carmem (Muscat Belly A × BRS Rúbea) Grapes: Evaluation of Their Potential Use as Bioingredients. Foods. 2023; 12(13):2608. https://doi.org/10.3390/foods12132608
Chicago/Turabian StyleNishiyama-Hortense, Yara Paula, Carolina Olivati, José Pérez-Navarro, Reginaldo Teodoro Souza, Natália S. Janzantti, Roberto Da-Silva, Isidro Hermosín-Gutiérrez, Sergio Gómez-Alonso, and Ellen Silva Lago-Vanzela. 2023. "Phenolic Composition of Brazilian BRS Carmem (Muscat Belly A × BRS Rúbea) Grapes: Evaluation of Their Potential Use as Bioingredients" Foods 12, no. 13: 2608. https://doi.org/10.3390/foods12132608
APA StyleNishiyama-Hortense, Y. P., Olivati, C., Pérez-Navarro, J., Souza, R. T., Janzantti, N. S., Da-Silva, R., Hermosín-Gutiérrez, I., Gómez-Alonso, S., & Lago-Vanzela, E. S. (2023). Phenolic Composition of Brazilian BRS Carmem (Muscat Belly A × BRS Rúbea) Grapes: Evaluation of Their Potential Use as Bioingredients. Foods, 12(13), 2608. https://doi.org/10.3390/foods12132608