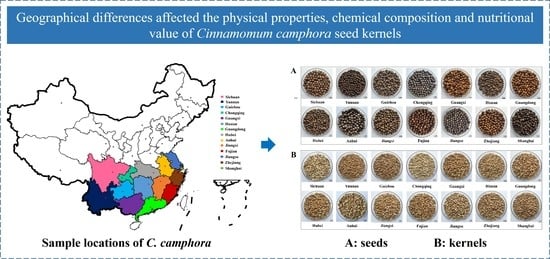
Geographic Pattern of Variations in Chemical Composition and Nutritional Value of Cinnamomum camphora Seed Kernels from China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Chemical Reagents
2.3. Measurement of Fruit, Seed and Kernel Weights
2.4. Determination of Proximate Composition of CCSK
2.5. Determination of Minerals and Heavy Metals of CCSK
2.6. Determination of Phytochemical Constituents of CCSKs
2.6.1. Preparation of Phytochemical Extracts
2.6.2. Total Phenolic Content (TPC)
2.6.3. Total Flavonoid Content (TFC)
2.6.4. HPLC Profile
2.7. Determination of In Vitro Antioxidant Activity
2.8. Determination of Fatty Acid Composition in Kernel Oil of CCSKs
2.9. Determination of Amino Acid Composition in Kernel Protein of CCSKs
2.10. Protein Profiles
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics
3.1.1. Shape and Color
3.1.2. Weights
3.2. Proximate Composition
3.3. Minerals and Heavy Metals
3.4. Phytochemical Composition
3.5. Antioxidant Activity
3.6. Fatty Acid Composition
3.7. Amino Acid Composition
3.8. Protein Profile
3.9. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, Y.; Ponnampalam, E.N.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Extraction and characterization of polyphenols from non-conventional edible plants and their antioxidant activities. Food Res. Int. 2022, 157, 111205. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, X.; Wu, S.; Ma, M.; Yu, P.; Gong, D.; Deng, S.; Zeng, Z. Ethanol extracts from Cinnamomum camphora seed kernel: Potential bioactivities as affected by alkaline hydrolysis and simulated gastrointestinal digestion. Food Res. Int. 2020, 137, 109363. [Google Scholar] [CrossRef]
- Yan, X.; Liang, S.; Peng, T.; Zhang, G.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Influence of phenolic compounds on physicochemical and functional properties of protein isolate from Cinnamomum camphora seed kernel. Food Hydrocoll. 2020, 102, 105612. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, B.; Li, S.; Zou, Z. Optimization of solvent-free microwave assisted extraction of essential oil from Cinnamomum camphora leaves. Ind. Crops Prod. 2018, 124, 353–362. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Zhu, Z.; Huang, D.; Qi, Y.; Ma, C.; Zou, Z.; Ni, H. Cinnamomum camphora fruit peel as a source of essential oil extracted using the solvent-free microwave-assisted method compared with conventional hydrodistillation. LWT 2022, 153, 112549. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Chanotiya, C.S.; Alam, M.; Samad, A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crops Prod. 2013, 49, 628–633. [Google Scholar] [CrossRef]
- Pandey, A.K.; Bora, H.R.; Deka, S.C.; Rastogi, R.C.; Baruah, A.K.S. Composition of the essential oil of the bark of Cinnamomum camphora. J. Med. Aromat. Plant Sci. 1997, 19, 408–409. [Google Scholar]
- Wang, P.; Wan, D.; Peng, T.; Yang, Y.; Wen, X.; Yan, X.; Xia, J.; Zhu, Q.; Yu, P.; Gong, D.; et al. Acute oral toxicity and genotoxicity test and evaluation of Cinnamomum camphora seed kernel oil. Foods 2023, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, B.; Gong, D.; Zeng, C.; Jiang, Y.; Zeng, Z. Camphor tree seed kernel oil reduces body fat deposition and improves blood lipids in rats. J. Food Sci. 2015, 80, H1912–H1917. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, C.; Zeng, Z.; Wang, B.; Gong, D. Cinnamomum camphora seed kernel oil ameliorates oxidative stress and inflammation in diet-induced obese rats. J. Food Sci. 2016, 81, H1295–H1300. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, X.; Xia, J.; Zhao, J.; Ma, M.; Yu, P.; Gong, D.; Zeng, Z. Assessment of the effect of ethanol extracts from Cinnamomum camphora seed kernel on intestinal inflammation using simulated gastrointestinal digestion and a Caco-2/RAW264.7 co-culture system. Food Funct. 2021, 12, 9197–9210. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, L.-N.; Wang, X.-S.; Gao, J.-Y.; Yi, J.-P.; Deng, R.-X. Characterization of Paeonia ostii seed and oil sourced from different cultivation areas in China. Ind. Crops Prod. 2019, 133, 63–71. [Google Scholar] [CrossRef]
- Wu, Z.; Meenu, M.; Xu, B. Nutritional value and antioxidant activity of Chinese black truffle (Tuber indicum) grown in different geographical regions in China. LWT 2021, 135, 110226. [Google Scholar] [CrossRef]
- Qiao, Q.; Hu, K.; Wu, C.; Wang, J.; Liu, Q.; Tao, J.; Liu, Y.; Lu, Y.; Feng, Z. Acer triflorum as a new nervonic acid-containing oilseed resource for the future: Fruit-and-seed weight, oil content and fatty acid composition. Ind. Crops Prod. 2022, 179, 114684. [Google Scholar] [CrossRef]
- Tian, W.; Cheng, D.; Yan, X.; Zhang, G.; Zeng, G.; Bao, X.; Zeng, Z.; Yu, P.; Gong, D. Effect of in vitro digestion of Cudrania cochinchinensis root extracts on phenolic compounds, bioactivity, bioaccessibility and cytotoxicity on HepG2 cells. Eur. Food Res. Technol. 2021, 247, 2945–2959. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, G.; Zhao, J.; Ma, M.; Bao, X.; Zeng, Z.; Gong, X.; Yu, P.; Wen, X.; Gong, D. Influence of phenolic compounds on the structural characteristics, functional properties and antioxidant activities of Alcalase-hydrolyzed protein isolate from Cinnamomum camphora seed kernel. LWT 2021, 148, 111799. [Google Scholar] [CrossRef]
- Ye, G.; Wu, Y.; Wang, L.; Tan, B.; Shen, W.; Li, X.; Liu, Y.; Tian, X.; Zhang, D. Comparison of six modification methods on the chemical composition, functional properties and antioxidant capacity of wheat bran. LWT 2021, 149, 111996. [Google Scholar] [CrossRef]
- Zeng, G.; Tian, W.; Zeng, Z.; Yan, X.; Yu, P.; Gong, D.; Wang, J. Construction and in vitro digestibility evaluation of a novel human milk fat substitute rich in structured triglycerides. Food Sci. Technol. 2022, 42, e10422. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef]
- Pal, R.S.; Bhartiya, A.; ArunKumar, R.; Kant, L.; Aditya, J.P.; Bisht, J.K. Impact of dehulling and germination on nutrients, antinutrients, and antioxidant properties in horsegram. J. Food Sci. Technol. 2016, 53, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Zhao, R.; Wen, X.; Yu, P.; Zeng, Z.; Deng, S.; Gong, D. Screening and identification of a Bacillus amyloliquefaciens strain for aqueous enzymatic extraction of medium-chain triglycerides. Food Control 2017, 78, 24–32. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, C.; Zeng, Z.; Wang, B.; Wen, X.; Yu, P.; Gong, D. Cinnamomum camphora seed Kernel oil improves lipid metabolism and enhances β3-adrenergic receptor expression in diet-induced obese rats. Lipids 2016, 51, 693–702. [Google Scholar] [CrossRef]
- Al-Farga, A.; Zhang, H.; Siddeeg, A.; Shamoon, M.; Chamba, M.V.; Al-Hajj, N. Proximate composition, functional properties, amino acid, mineral and vitamin contents of a novel food: Alhydwan (Boerhavia elegana Choisy) seed flour. Food Chem. 2016, 211, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kamila, P.K.; Bal, P.; Ray, A.; Kar, S.K.; Panda, P.C. Nutritional value, phytochemical composition and antioxidant potential of the seed flour of Cycas sphaerica, endemic to India. S. Afr. J. Bot. 2022, 150, 965–973. [Google Scholar] [CrossRef]
- Wang, P.; Shan, N.; Ali, A.; Sun, J.; Luo, S.; Xiao, Y.; Wang, S.; Hu, R.; Huang, Y.; Zhou, Q. Comprehensive evaluation of functional components, biological activities, and minerals of yam species (Dioscorea polystachya and D. alata) from China. LWT 2022, 168, 113964. [Google Scholar] [CrossRef]
- Lozano Muñoz, I.; Díaz, N.F. Minerals in edible seaweed: Health benefits and food safety issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 1592–1607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Meng, L.; Wang, X.; Zhao, W.; Shi, X.; Wang, W.; Li, Z.; Wang, L. The yield, nutritional value, umami components and mineral contents of the first-flush and second-flush Pleurotus pulmonarius mushrooms grown on three forestry wastes. Food Chem. 2022, 397, 133714. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhou, X.; Chen, S.; Sun, Y.; Shen, Y.; Ye, X. Chemical composition and antioxidant activity of Chinese wild raspberry (Rubus hirsutus Thunb.). LWT 2015, 60, 1262–1268. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.X.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Effect of domestic cooking on carotenoids, tocopherols, fatty acids, phenolics, and antioxidant activities of lentils (Lens culinaris). J. Agric. Food Chem. 2014, 62, 12585–12594. [Google Scholar] [CrossRef]
- Yilmaz, V.A.; Brandolini, A.; Hidalgo, A. Phenolic acids and antioxidant activity of wild, feral and domesticated diploid wheats. J. Cereal Sci. 2015, 64, 168–175. [Google Scholar] [CrossRef]
- Hu, J.-N.; Zhang, B.; Zhu, X.-M.; Li, J.; Fan, Y.-W.; Liu, R.; Tang, L.; Lee, K.-T.; Deng, Z.-Y. Characterization of medium-chain triacylglycerol (MCT)-enriched seed oil from Cinnamomum camphora (Lauraceae) and its oxidative stability. J. Agric. Food Chem. 2011, 59, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yu, B.; Curran, P.; Liu, S.-Q. Lipase-catalysed transesterification of coconut oil with fusel alcohols in a solvent-free system. Food Chem. 2012, 134, 89–94. [Google Scholar] [CrossRef]
- Kılıç, B.; Özer, C.O. Potential use of interesterified palm kernel oil to replace animal fat in frankfurters. Meat Sci. 2019, 148, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hu, Z.; Mao, J.; Xu, Y.; Zhu, X.; Xiong, H. Synthesis of cocoa butter substitutes from Cinnamomum camphora seed oil and fully hydrogenated palm oil by enzymatic interesterification. J. Food Sci. Technol. 2019, 56, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, X.; Ma, X.; Xiong, H.; Zeng, Z.; Peng, H.; Hu, J. Enzymatic production of trans-free shortening from coix seed oil, fully hydrogenated palm oil and Cinnamomum camphora seed oil. Food Biosci. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, J.; Zhu, X.; Li, H.; Li, J.; Fan, Y.; Deng, Z. Enzymatic synthesis of medium- and long-chain triacylglycerols–enriched structured lipid from Cinnamomum camphora seed oil and camellia oil by Lipozyme RM IM. Int. J. Food Sci. Technol. 2014, 49, 453–459. [Google Scholar] [CrossRef]
- Bonke, A.; Sieuwerts, S.; Petersen, I.L. Amino acid composition of novel plant drinks from oat, lentil and pea. Foods 2020, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Chen, J.; Sun, B.; Wang, Z.; Zhu, J.; Yue, Z.; Zhang, Y.; Shan, A.; Ma, Q.; Wang, J. Leucine, but not isoleucine or valine, affects serum lipid profiles and browning of WAT in mice. Food Funct. 2021, 12, 6712–6724. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Li, D.; Gu, Z. Evaluation of sugar, free amino acid, and organic acid compositions of different varieties of vegetable soybean (Glycine max [L.] Merr). Ind. Crops Prod. 2013, 50, 743–749. [Google Scholar] [CrossRef]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed protein of lentils: Current status, progress, and food applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Chee, K.L.; Ling, H.K.; Ayob, M.K. Optimization of trypsin-assisted extraction, physico-chemical characterization, nutritional qualities and functionalities of palm kernel cake protein. LWT 2012, 46, 419–427. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Wang, B.; Adhikari, B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016, 197, 212–220. [Google Scholar] [CrossRef]
- Egydio, A.P.M.; Catarina, C.S.; Floh, E.I.S.; Santos, D.Y.A.C.d. Free amino acid composition of Annona (Annonaceae) fruit species of economic interest. Ind. Crops Prod. 2013, 45, 373–376. [Google Scholar] [CrossRef]
- Yan, X.; Gao, Y.; Liu, S.; Zhang, G.; Zhao, J.; Cheng, D.; Zeng, Z.; Gong, X.; Yu, P.; Gong, D. Covalent modification by phenolic extract improves the structural properties and antioxidant activities of the protein isolate from Cinnamomum camphora seed kernel. Food Chem. 2021, 352, 129377. [Google Scholar] [CrossRef] [PubMed]





| No. | Geographical Regions | Coordinates | ||
|---|---|---|---|---|
| Province | City | County/District | ||
| 1 | Sichuan | Chengdu | Wuhou | 104°06′ E/30°38′ N |
| 2 | Yunnan | Qujing | Xuanwei | 104°08′ E/26°14′ N |
| 3 | Guizhou | Bijie | Nayong | 105°23′ E/26°47′ N |
| 4 | Chongqing | Chongqing | Shapingba | 106°29′ E/29°34′ N |
| 5 | Guangxi | Chongzuo | Jiangzhou | 107°24′ E/22°24′ N |
| 6 | Hunan | Changsha | Tianxin | 113°02′ E/28°08′ N |
| 7 | Guangdong | Shenzhen | Bao’an | 113°56′ E/22°42′ N |
| 8 | Hubei | Xianning | Xian’an | 114°18′ E/29°52′ N |
| 9 | Anhui | Fuyang | Yingdong | 115°56′ E/32°52′ N |
| 10 | Jiangxi | Nanchang | Qingshanhu | 115°58′ E/28°40′ N |
| 11 | Fujian | Xiamen | Huli | 118°08′ E/24°30′ N |
| 12 | Jiangsu | Nanjing | Jianye | 118°46′ E/32°02′ N |
| 13 | Zhejiang | Wenzhou | Rui’an | 120°28′ E/27°50′ N |
| 14 | Shanghai | Shanghai | Pudong | 121°33′ E/31°09′ N |
| Sample | W1 (g) | W2 (g) | W3 (g) | W4 (g) | R1 a (%) | R2 (%) |
|---|---|---|---|---|---|---|
| Sichuan | 650.90 ± 9.17 cd | 241.47 ± 4.97 b | 155.47 ± 4.01 cd | 114.04 ± 2.54 a | 37.10 ± 0.75 abc | 73.36 ± 0.54 bc |
| Yunnan | 387.80 ± 6.61 g | 114.57 ± 5.54 g | 74.35 ± 1.11 i | 42.97 ± 0.74 h | 29.53 ± 0.99 f | 57.80 ± 1.54 efgh |
| Guizhou | 453.77 ± 8.56 f | 160.07 ± 3.36 e | 115.77 ± 4.64 g | 92.25 ± 1.89 e | 35.28 ± 0.69 bcde | 79.76 ± 3.37 a |
| Chongqing | 562.33 ± 20.60 e | 202.67 ± 7.64 c | 145.23 ± 5.65 de | 81.15 ± 1.57 f | 36.04 ± 0.77 bcde | 55.90 ± 1.10 fgh |
| Guangxi | 761.90 ± 7.64 b | 262.90 ± 9.40 a | 189.60 ± 7.10 a | 99.34 ± 0.95 bcd | 34.51 ± 1.29 e | 52.46 ± 2.47 hi |
| Hunan | 339.03 ± 9.58 h | 122.53 ± 7.20 fg | 81.77 ± 1.36 i | 61.48 ± 0.90 g | 36.12 ± 1.14 bcde | 75.19 ± 0.92 abc |
| Guangdong | 896.87 ± 14.89 a | 250.17 ± 6.63 ab | 171.73 ± 3.06 b | 81.68 ± 1.18 f | 27.89 ± 0.35 f | 47.57 ± 0.56 i |
| Hubei | 477.53 ± 8.32 f | 134.20 ± 2.52 f | 101.73 ± 3.56 h | 62.44 ± 0.55 g | 28.11 ± 0.58 f | 61.41 ± 1.62 def |
| Anhui | 550.50 ± 23.48 e | 215.45 ± 4.52 c | 146.67 ± 5.38 de | 92.03 ± 2.08 e | 39.15 ± 0.87 a | 62.77 ± 0.91 de |
| Jiangxi | 536.07 ± 17.26 e | 183.03 ± 7.12 d | 131.90 ± 2.26 f | 100.99 ± 2.19 b | 34.14 ± 0.27 de | 76.57 ± 1.62 ab |
| Fujian | 692.07 ± 11.55 c | 240.20 ± 3.50 b | 164.13 ± 3.98 bc | 95.67 ± 0.98 cde | 34.71 ± 0.47 cde | 58.32 ± 1.99 defg |
| Jiangsu | 539.07 ± 16.73 e | 201.30 ± 2.21 c | 136.13 ± 4.90 ef | 95.00 ± 1.44 de | 37.36 ± 0.85 ab | 69.82 ± 1.51 c |
| Zhejiang | 645.43 ± 18.90 d | 236.33 ± 6.35 b | 157.37 ± 3.39 cd | 100.05 ± 3.28 bc | 36.62 ± 0.23 abcd | 63.60 ± 2.57 d |
| Shanghai | 477.03 ± 13.85 f | 171.93 ± 7.03 de | 123.80 ± 3.56 fg | 65.33 ± 2.46 g | 36.03 ± 0.43 bcde | 52.84 ± 3.55 ghi |
| Sample | Moisture | Oil | Protein | Dietary Fiber | Ash |
|---|---|---|---|---|---|
| Sichuan | 2.43 ± 0.06 def | 53.90 ± 1.15 bcde | 17.86 ± 0.23 c | 6.65 ± 0.21 de | 2.32 ± 0.09 efg |
| Yunnan | 2.35 ± 0.08 ef | 54.83 ± 1.85 bcde | 12.33 ± 0.32 f | 6.93 ± 0.27 cde | 2.24 ± 0.08 fgh |
| Guizhou | 1.94 ± 0.07 g | 57.89 ± 1.63 ab | 14.38 ± 0.22 f | 6.92 ± 0.19 cde | 2.08 ± 0.04 h |
| Chongqing | 3.28 ± 0.12 a | 62.08 ± 2.13 a | 18.36 ± 0.27 c | 5.66 ± 0.18 f | 2.53 ± 0.06 cd |
| Guangxi | 2.32 ± 0.05 ef | 54.02 ± 1.48 bcde | 19.48 ± 0.21 b | 7.13 ± 0.34 bcde | 2.30 ± 0.06 efg |
| Hunan | 2.32 ± 0.04 ef | 46.29 ± 1.30 f | 16.53 ± 0.25 e | 6.95 ± 0.08 cde | 2.38 ± 0.04 def |
| Guangdong | 2.26 ± 0.08 f | 50.33 ± 1.64 ef | 20.07 ± 0.40 b | 7.58 ± 0.41 abc | 2.15 ± 0.04 gh |
| Hubei | 2.89 ± 0.07 b | 55.79 ± 1.40 bc | 17.81 ± 0.16 c | 6.91 ± 0.26 cde | 2.63 ± 0.08 bc |
| Anhui | 2.79 ± 0.11 bc | 61.79 ± 0.84 a | 16.93 ± 0.37 d | 6.36 ± 0.12 ef | 2.29 ± 0.05 efg |
| Jiangxi | 1.62 ± 0.08 h | 53.15 ± 1.18 cde | 18.35 ± 0.14 d | 7.84 ± 0.22 ab | 2.96 ± 0.08 a |
| Fujian | 2.55 ± 0.11 de | 55.27 ± 2.18 bcd | 16.31 ± 0.33 d | 8.03 ± 0.30 a | 2.45 ± 0.06 cde |
| Jiangsu | 2.87 ± 0.05 b | 50.93 ± 1.66 def | 19.83 ± 0.38 b | 7.31 ± 0.17 abcd | 2.41 ± 0.06 def |
| Zhejiang | 2.58 ± 0.06 cd | 56.88 ± 1.52 bc | 18.11 ± 0.32 c | 7.07 ± 0.21 bcde | 2.31 ± 0.03 efg |
| Shanghai | 3.00 ± 0.09 b | 51.04 ± 1.08 def | 22.17 ± 0.15 a | 7.80 ± 0.43 ab | 2.76 ± 0.09 b |
| Sample | Ca | K | Mg | Na | P | Mn | Fe | Cu | Zn | As | Se | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sichuan | 2895.71 | 4347.44 | 2158.52 | 97.68 | 3213.95 | 325.95 | 47.93 | 39.76 | 10.85 | 0.01 | 0.50 | 0.05 | 0.03 | 0.16 |
| Yunnan | 2726.93 | 7171.88 | 3147.32 | 161.68 | 2735.86 | 11.69 | 17.90 | 4.33 | 7.58 | 0.00 | 0.12 | 0.02 | 0.04 | 0.08 |
| Guizhou | 1412.27 | 4798.14 | 2298.14 | 140.37 | 3382.76 | 220.01 | 53.92 | 27.53 | 9.92 | 0.02 | 0.33 | 0.07 | 0.00 | 0.08 |
| Chongqing | 1632.25 | 7186.89 | 2651.85 | 253.96 | 4116.05 | 30.26 | 75.76 | 27.52 | 9.45 | 0.01 | 0.56 | 0.02 | 0.00 | 0.07 |
| Guangxi | 1661.46 | 4345.05 | 2028.65 | 150.98 | 4195.96 | 20.87 | 39.49 | 30.23 | 13.03 | 0.01 | 0.40 | 0.02 | 0.01 | 0.13 |
| Hunan | 4573.18 | 6307.44 | 2996.73 | 120.77 | 4545.38 | 115.34 | 60.15 | 43.07 | 16.75 | 0.01 | 0.76 | 0.04 | 0.01 | 0.13 |
| Guangdong | 3264.21 | 4667.47 | 2457.68 | 237.18 | 4230.96 | 139.66 | 41.62 | 40.29 | 53.07 | 0.01 | 0.56 | 0.05 | 0.00 | 0.08 |
| Hubei | 2793.24 | 5252.61 | 2976.67 | 106.36 | 5385.36 | 20.72 | 43.39 | 43.31 | 23.27 | 0.01 | 0.53 | 0.01 | 0.01 | 0.16 |
| Anhui | 1975.02 | 7052.99 | 2369.42 | 173.43 | 4068.13 | 106.35 | 48.46 | 1.29 | 5.62 | 0.15 | 0.08 | 0.04 | 0.00 | 0.29 |
| Jiangxi | 2915.32 | 6534.95 | 2703.63 | 113.17 | 4385.75 | 14.97 | 23.93 | 15.40 | 16.19 | 0.00 | 0.11 | 0.02 | 0.01 | 0.07 |
| Fujian | 2666.44 | 5067.79 | 3128.19 | 106.98 | 4009.40 | 83.22 | 65.30 | 22.83 | 17.82 | 0.01 | 0.41 | 0.04 | 0.00 | 0.11 |
| Jiangsu | 2063.66 | 6350.85 | 2563.29 | 107.99 | 4033.31 | 13.86 | 54.56 | 32.75 | 11.48 | 0.01 | 0.45 | 0.04 | 0.01 | 0.14 |
| Zhejiang | 2867.80 | 4538.61 | 2198.30 | 105.24 | 4094.90 | 123.71 | 48.40 | 19.68 | 14.86 | 0.01 | 0.24 | 0.10 | 0.00 | 0.05 |
| Shanghai | 3327.37 | 6301.79 | 2869.57 | 181.24 | 5211.42 | 31.70 | 68.61 | 48.42 | 19.28 | 0.01 | 1.63 | 0.02 | 0.01 | 0.15 |
| Sample | C8:0 | C10:0 | C12:0 | C14:0 | C16:0 | C18:0 | C18:1 | C18:2 | C20:0 | SFA a | USFA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sichuan | 0.35 ± 0.03 ef | 58.37 ± 0.66 abcd | 37.75 ± 0.63 ab | 0.73 ± 0.01 c | 0.16 ± 0.02 bcd | 0.07 ± 0.01 a | 2.51 ± 0.07 a | 0.17 ± 0.03 b | 0.00 ± 0.00 c | 97.43 ± 1.22 a | 2.68 ± 0.10 ab |
| Yunnan | 0.44 ± 0.02 ab | 60.18 ± 0.50 a | 35.23 ± 0.84 c | 0.83 ± 0.12 abc | 0.13 ± 0.01 d | 0.06 ± 0.01 a | 2.37 ± 0.07 a | 0.24 ± 0.03 ab | 0.00 ± 0.00 c | 96.88 ± 1.40 a | 2.61 ± 0.09 ab |
| Guizhou | 0.33 ± 0.01 f | 58.09 ± 0.36 bcd | 37.32 ± 0.48 abc | 0.78 ± 0.01 bc | 0.15 ± 0.01 cd | 0.07 ± 0.01 a | 2.54 ± 0.12 a | 0.26 ± 0.03 a | 0.03 ± 0.01 a | 96.77 ± 0.88 a | 2.80 ± 0.15 a |
| Chongqing | 0.42 ± 0.03 abcd | 58.98 ± 1.02 abcd | 36.81 ± 0.69 abc | 0.73 ± 0.02 c | 0.16 ± 0.01 abcd | 0.06 ± 0.01 a | 2.34 ± 0.12 a | 0.18 ± 0.06 b | 0.00 ± 0.00 c | 97.16 ± 1.05 a | 2.52 ± 0.16 b |
| Guangxi | 0.46 ± 0.01 a | 59.86 ± 0.79 ab | 35.71 ± 0.65 bc | 0.81 ± 0.08 abc | 0.16 ± 0.01 bcd | 0.00 ± 0.00 b | 2.43 ± 0.15 a | 0.23 ± 0.01 ab | 0.00 ± 0.00 c | 96.99 ± 0.86 a | 2.66 ± 0.16 ab |
| Hunan | 0.42 ± 0.01 abcd | 57.68 ± 0.31 cd | 36.51 ± 0.33 abc | 0.86 ± 0.03 abc | 0.16 ± 0.01 abcd | 0.05 ± 0.01 a | 2.46 ± 0.05 a | 0.19 ± 0.02 ab | 0.03 ± 0.01 a | 95.71 ± 1.08 a | 2.65 ± 0.06 ab |
| Guangdong | 0.40 ± 0.02 bcde | 58.15 ± 0.62 bcd | 37.62 ± 0.65 ab | 0.91 ± 0.04 ab | 0.17 ± 0.02 abcd | 0.05 ± 0.01 a | 2.39 ± 0.04 a | 0.20 ± 0.03 ab | 0.03 ± 0.01 a | 97.33 ± 1.32 a | 2.59 ± 0.07 ab |
| Hubei | 0.44 ± 0.01 abc | 58.84 ± 0.49 abcd | 36.98 ± 0.64 abc | 0.91 ± 0.07 ab | 0.19 ± 0.02 abc | 0.03 ± 0.00 ab | 2.51 ± 0.05 a | 0.19 ± 0.01 ab | 0.00 ± 0.00 c | 97.39 ± 1.17 a | 2.71 ± 0.07 ab |
| Anhui | 0.38 ± 0.03 cdef | 58.07 ± 1.61 bcd | 37.17 ± 0.77 abc | 0.80 ± 0.03 bc | 0.15 ± 0.02 cd | 0.07 ± 0.01 a | 2.52 ± 0.09 a | 0.22 ± 0.02 ab | 0.00 ± 0.00 c | 96.64 ± 1.35 a | 2.74 ± 0.10 ab |
| Jiangxi | 0.36 ± 0.01 def | 58.41 ± 0.16 abcd | 37.42 ± 0.15 abc | 0.92 ± 0.09 ab | 0.14 ± 0.02 d | 0.06 ± 0.01 a | 2.42 ± 0.06 a | 0.21 ± 0.01 ab | 0.00 ± 0.00 c | 97.34 ± 1.26 a | 2.63 ± 0.08 ab |
| Fujian | 0.44 ± 0.03 abc | 59.44 ± 0.23 abc | 36.09 ± 0.24 abc | 0.84 ± 0.03 abc | 0.20 ± 0.01 a | 0.08 ± 0.01 a | 2.46 ± 0.06 a | 0.21 ± 0.01 ab | 0.03 ± 0.01 a | 97.09 ± 0.96 a | 2.67 ± 0.07 ab |
| Jiangsu | 0.42 ± 0.01 abcd | 59.16 ± 0.31 abcd | 35.85 ± 1.48 bc | 0.76 ± 0.06 bc | 0.14 ± 0.02 cd | 0.06 ± 0.01 a | 2.40 ± 0.08 a | 0.20 ± 0.02 ab | 0.00 ± 0.00 c | 96.40 ± 1.03 a | 2.60 ± 0.11 ab |
| Zhejiang | 0.38 ± 0.01 def | 57.37 ± 0.73 d | 38.29 ± 0.69 a | 0.98 ± 0.08 a | 0.19 ± 0.01 ab | 0.00 ± 0.00 b | 2.51 ± 0.07 a | 0.22 ± 0.02 ab | 0.00 ± 0.00 c | 97.20 ± 0.97 a | 2.73 ± 0.08 ab |
| Shanghai | 0.35 ± 0.02 ef | 59.66 ± 0.26 abc | 36.47 ± 1.05 abc | 0.82 ± 0.06 abc | 0.15 ± 0.02 bcd | 0.06 ± 0.01 a | 2.43 ± 0.06 a | 0.22 ± 0.01 ab | 0.01 ± 0.00 b | 97.52 ± 1.14 a | 2.65 ± 0.06 ab |
| Sample | Val | Leu | Ile | Phe | Met | Lys | His | Thr | TEAA a | Cys | Asp | Ser | Glu | Gly | Arg | Pro | Ala | Tyr | TAA | TEAA/TAA (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sichuan | 0.35 | 1.06 | 0.34 | 0.76 | 0.21 | 0.81 | 0.28 | 0.43 | 4.23 | 0.77 | 1.48 | 0.84 | 2.55 | 0.71 | 1.59 | 0.67 | 0.67 | 0.63 | 14.15 | 29.89 |
| Yunnan | 0.47 | 0.92 | 0.47 | 0.59 | 0.11 | 0.69 | 0.23 | 0.40 | 3.87 | 0.51 | 1.05 | 0.61 | 1.87 | 0.50 | 1.18 | 0.46 | 0.51 | 0.47 | 11.04 | 35.05 |
| Guizhou | 0.49 | 0.96 | 0.43 | 0.63 | 0.17 | 0.76 | 0.24 | 0.50 | 4.17 | 0.61 | 1.07 | 0.69 | 1.92 | 0.47 | 1.08 | 0.43 | 0.60 | 0.47 | 11.52 | 36.20 |
| Chongqing | 0.32 | 0.99 | 0.29 | 0.71 | 0.20 | 0.85 | 0.27 | 0.38 | 4.00 | 0.83 | 1.53 | 0.76 | 2.52 | 0.71 | 1.57 | 0.66 | 0.67 | 0.60 | 13.87 | 28.84 |
| Guangxi | 0.39 | 1.10 | 0.32 | 0.77 | 0.23 | 0.91 | 0.27 | 0.44 | 4.43 | 0.90 | 1.48 | 0.82 | 2.51 | 0.72 | 1.70 | 0.73 | 0.67 | 0.63 | 14.60 | 30.34 |
| Hunan | 0.35 | 1.14 | 0.39 | 0.80 | 0.21 | 0.90 | 0.32 | 0.47 | 4.58 | 0.75 | 1.58 | 0.92 | 2.72 | 0.75 | 1.70 | 0.70 | 0.72 | 0.67 | 15.08 | 30.37 |
| Guangdong | 0.42 | 1.30 | 0.44 | 0.87 | 0.24 | 0.87 | 0.31 | 0.51 | 4.96 | 0.91 | 1.66 | 0.99 | 3.00 | 0.79 | 1.89 | 0.79 | 0.75 | 0.72 | 16.48 | 30.10 |
| Hubei | 0.34 | 1.01 | 0.31 | 0.69 | 0.20 | 0.77 | 0.25 | 0.42 | 3.99 | 0.76 | 1.37 | 0.76 | 2.28 | 0.65 | 1.51 | 0.65 | 0.63 | 0.55 | 13.16 | 30.32 |
| Anhui | 0.33 | 1.04 | 0.34 | 0.70 | 0.18 | 0.88 | 0.27 | 0.42 | 4.16 | 0.79 | 1.44 | 0.72 | 2.41 | 0.68 | 1.45 | 0.67 | 0.66 | 0.54 | 13.53 | 30.75 |
| Jiangxi | 0.73 | 1.30 | 0.68 | 0.85 | 0.12 | 1.12 | 0.32 | 0.57 | 5.68 | 1.00 | 1.53 | 0.85 | 2.72 | 0.70 | 1.86 | 0.73 | 0.69 | 0.73 | 16.48 | 34.47 |
| Fujian | 0.32 | 1.02 | 0.35 | 0.66 | 0.20 | 0.82 | 0.25 | 0.43 | 4.05 | 0.79 | 1.32 | 0.79 | 2.38 | 0.63 | 1.48 | 0.67 | 0.60 | 0.55 | 13.26 | 30.54 |
| Jiangsu | 0.36 | 1.09 | 0.33 | 0.76 | 0.23 | 0.87 | 0.28 | 0.45 | 4.37 | 0.92 | 1.53 | 0.86 | 2.55 | 0.73 | 1.63 | 0.74 | 0.69 | 0.62 | 14.65 | 29.83 |
| Zhejiang | 0.32 | 1.00 | 0.31 | 0.70 | 0.21 | 0.81 | 0.25 | 0.41 | 4.01 | 0.79 | 1.35 | 0.77 | 2.32 | 0.66 | 1.46 | 0.66 | 0.63 | 0.57 | 13.22 | 30.33 |
| Shanghai | 0.43 | 1.35 | 0.43 | 0.93 | 0.24 | 1.00 | 0.35 | 0.53 | 5.26 | 0.78 | 1.81 | 1.04 | 3.09 | 0.86 | 2.01 | 0.79 | 0.82 | 0.79 | 17.25 | 30.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Gong, X.; Zeng, Z.; Xia, J.; Ma, M.; Zhao, J.; Zhang, G.; Wang, P.; Wan, D.; Yu, P.; et al. Geographic Pattern of Variations in Chemical Composition and Nutritional Value of Cinnamomum camphora Seed Kernels from China. Foods 2023, 12, 2630. https://doi.org/10.3390/foods12132630
Yan X, Gong X, Zeng Z, Xia J, Ma M, Zhao J, Zhang G, Wang P, Wan D, Yu P, et al. Geographic Pattern of Variations in Chemical Composition and Nutritional Value of Cinnamomum camphora Seed Kernels from China. Foods. 2023; 12(13):2630. https://doi.org/10.3390/foods12132630
Chicago/Turabian StyleYan, Xianghui, Xiaofeng Gong, Zheling Zeng, Jiaheng Xia, Maomao Ma, Junxin Zhao, Guohua Zhang, Pengbo Wang, Dongman Wan, Ping Yu, and et al. 2023. "Geographic Pattern of Variations in Chemical Composition and Nutritional Value of Cinnamomum camphora Seed Kernels from China" Foods 12, no. 13: 2630. https://doi.org/10.3390/foods12132630
APA StyleYan, X., Gong, X., Zeng, Z., Xia, J., Ma, M., Zhao, J., Zhang, G., Wang, P., Wan, D., Yu, P., & Gong, D. (2023). Geographic Pattern of Variations in Chemical Composition and Nutritional Value of Cinnamomum camphora Seed Kernels from China. Foods, 12(13), 2630. https://doi.org/10.3390/foods12132630