The Development of Highly pH-Sensitive Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films Loaded with Anthocyanin/Curcumin for the Fresh-Keeping and Freshness Detection of Fresh Pork
Abstract
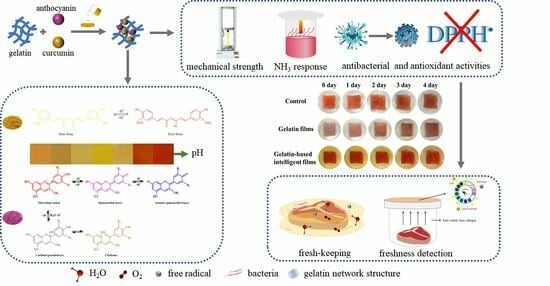
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Bacterial Cellulose Nanofibers (BCNs)
2.3. Preparation of Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films
2.4. UV–Vis Absorption Spectra of Natural Pigment Solutions at Different pH Values
2.5. Scanning Electron Microscopy (SEM)
2.6. Atomic Force Microscopy (AFM)
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. X-ray Diffraction (XRD)
2.9. Water Contact Angle (WCA)
2.10. Water Content (WC) and Water Solubility (WS)
2.11. Thickness, Water Vapor Permeability (WVP) and Relative Oxygen Transmission Rate (ROT)
2.12. Mechanical Properties
2.13. Transmittance and Opacity Measurements
2.14. Colorimetric Analysis
2.15. Determination of Antioxidant Property
2.16. pH Sensitivity of Intelligent Films
2.17. The Response of the Intelligent Films to Volatile Ammonia
2.18. Application of the Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films in the Packaging of Pork
2.19. Statistical Analysis
3. Results and Discussion
3.1. UV–Vis Spectra of Pigment Solutions at Different pH Values
3.2. Microstructure of Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films
3.3. Fourier Transfors Infrared (FTIR) Spectroscopy
3.4. X-ray Diffraction (XRD) Analysis
3.5. Water Contact Angle (WCA)
3.6. Thickness, Water Content (WC) and Water Solubility (WS) of Intelligent Films
3.7. Water Vapor Permeability (WVP) and Relative Oxygen Transmission Rate (ROT) of Intelligent Films
3.8. Mechanical Properties
3.9. Color and Transparency Properties of Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films
3.10. Antioxidant Property of Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films
3.11. pH Sensitivity of Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films
3.11.1. Color Response of Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films at Different pH Values
3.11.2. Color Response of Intelligent Films to Volatile Ammonia
3.12. Fresh-Keeping and Freshness Detection Properties of the Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films
3.12.1. Storage Quality of the Fresh Pork Packaged with the Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films
3.12.2. Pork Freshness Detection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Gopal, T.K.S. Smart packaging systems for food applications: A review. J. Food Sci. Technol.-Mysore 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Nuanmano, S.; Prodpran, T.; Benjakul, S. Potential use of gelatin hydrolysate as plasticizer in fish myofibrillar protein film. Food Hydrocoll. 2015, 47, 61–68. [Google Scholar] [CrossRef]
- Li, N.; Yang, X.; Lin, D. Development of bacterial cellulose nanofibers/konjac glucomannan-based intelligent films loaded with curcumin for the fresh-keeping and freshness monitoring of fresh beef. Food Packag. Shelf Life 2022, 34, 100989. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Emerging trends in food packaging. Nutr. Food Sci. 2018, 48, 764–779. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Gouveia Costa, H.L.; Lopes, D.R.; Prentice, C. Intelligent Packaging with pH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants trends and perspective. TrAC-Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Shen, N.; Ren, J.; Liu, Y.; Sun, W.; Li, Y.; Xin, H.; Cui, Y. Natural edible pigments: A comprehensive review of resource, chemical classification, biosynthesis pathway, separated methods and application. Food Chem. 2023, 403, 134422. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Almasi, H.; Forghani, S.; Moradi, M. Recent advances on intelligent food freshness indicators; an update on natural colorants and methods of preparation. Food Packag. Shelf Life 2022, 32, 100839. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives-A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yang, Y.J.; Kim, J.S.; Choi, D.S.; Park, S.H.; Jin, S.Y.; Park, J.S. Non-destructive monitoring of apple ripeness using an aldehyde sensitive colorimetric sensor. Food Chem. 2018, 267, 149–156. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Chen, H.-z.; Zhang, M.; Bhandari, B.; Yang, C.-H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. Int. J. Biol. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef]
- Zhai, X.C.; Lin, D.H.; Liu, D.J.; Yang, X.B. Emulsions stabilized by nanofibers from bacterial cellulose: New potential food-grade Pickering emulsions. Food Res. Int. 2018, 103, 12–20. [Google Scholar] [CrossRef]
- Sukhtezari, S.; Almasi, H.; Pirsa, S.; Zandi, M.; Pirouzifard, M. Development of bacterial cellulose based slow-release active films by incorporation of Scrophularia striata Boiss. extract. Carbohydr. Polym. 2017, 156, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, X.; Jin, R.; Li, D. Preparation and characterization of nanocomposite films containing starch and cellulose nanofibers. Ind. Crop. Prod. 2018, 123, 654–660. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Chen, M.; Ge, Y.; Ma, J.; Hu, Y.; Pang, J.; Yan, Z. Effect of oxidized chitin nanocrystals and curcumin into chitosan films for seafood freshness monitoring. Food Hydrocoll. 2019, 95, 308–317. [Google Scholar] [CrossRef]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhao, Y.; Shi, Q. Characterization of a novel edible film based on gum ghatti: Effect of plasticizer type and concentration. Carbohydr. Polym. 2016, 153, 345–355. [Google Scholar] [CrossRef]
- Ma, Q.; Cao, L.; Liang, T.; Li, J.; Lucia, L.A.; Wang, L. Active Tara Gum/PVA Blend Films with Curcumin-Loaded CTAC Brush-TEMPO-Oxidized Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2018, 6, 8926–8934. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; Xie, F.; Fan, Y.; Xu, X.; Qi, J.; Xiong, G.; Gao, X.; Zhang, F. pH-responsive double-layer indicator films based on konjac glucomannan/camellia oil and carrageenan/anthocyanin/curcumin for monitoring meat freshness. Food Hydrocoll. 2021, 118, 106695. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zou, X.; Shi, J.; Zhai, X.; Liu, L.; Li, Z.; Holmes, M.; Gong, Y.; Povey, M.; et al. A visual indicator based on curcumin with high stability for monitoring the freshness of freshwater shrimp, Macrobrachium rosenbergii. J. Food Eng. 2021, 292, 110290. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of Gelatin/Carrageenan-Based Color-Indicator Film Integrated with Shikonin and Propolis for Smart Food Packaging Applications. ACS Appl. Bio Mater. 2021, 4, 770–779. [Google Scholar] [CrossRef]
- Wang, X.; Guo, C.; Hao, W.; Ullah, N.; Chen, L.; Li, Z.; Feng, X. Development and characterization of agar-based edible films reinforced with nano-bacterial cellulose. Int. J. Biol. Macromol. 2018, 118, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.Y.; Jaiswal, S.; Jaiswal, A.K. A review on nanomaterials and nanohybrids based bio-nanocomposites for food packaging. Food Chem. 2022, 376, 131912. [Google Scholar] [CrossRef] [PubMed]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Use of gamma irradiation technology for modification of bacterial cellulose nanocrystals/chitosan nanocomposite film. Carbohydr. Polym. 2021, 253, 117144. [Google Scholar] [CrossRef]
- Dong, Y.T.; Rao, Z.L.; Liu, Y.C.; Zheng, X.J.; Tang, K.Y.; Liu, J. Soluble soybean polysaccharide/gelatin active edible films incorporated with curcumin for oil packaging. Food Packag. Shelf Life 2023, 35, 101039. [Google Scholar] [CrossRef]
- He, F.; Kong, Q.; Jin, Z.; Mou, H. Developing a unidirectionally permeable edible film based on kappa-carrageenan and gelatin for visually detecting the freshness of grass carp fillets. Carbohydr. Polym. 2020, 241, 116336. [Google Scholar] [CrossRef]
- Ni, P.; Bi, H.; Zhao, G.; Han, Y.; Wickramaratne, M.N.; Dai, H.; Wang, X. Electrospun preparation and biological properties in vitro of polyvinyl alcohol/sodium alginate/nano-hydroxyapatite composite fiber membrane. Colloids Surf. B Biointerfaces 2019, 173, 171–177. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Antioxidant and antimicrobial poly(vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Erna, K.H.; Felicia, W.X.L.; Vonnie, J.M.; Rovina, K.; Yin, K.W.; Nur’Aqilah, M.N. Synthesis and Physicochemical Characterization of Polymer Film-Based Anthocyanin and Starch. Biosensors 2022, 12, 211. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Physico-Mechanical Characterization and Antimicrobial Properties of Fish Protein Isolate/Fish Skin Gelatin-Zinc Oxide (ZnO) Nanocomposite Films. Food Bioprocess Technol. 2016, 9, 101–112. [Google Scholar] [CrossRef]
- Yang, S.; Li, H.; Sun, H. Preparation of gelatin-based films modified with nanocrystalline cellulose. Iran. Polym. J. 2018, 27, 645–652. [Google Scholar] [CrossRef]
- Shi, C.; Han, J.; Sun, X.; Guo, Y.; Yang, X.; Jia, Z. An intelligent colorimetric film based on complex anthocyanins and bacterial cellulose nanofibers for tilapia freshness detection in an actual cold chain. Int. J. Biol. Macromol. 2022, 221, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Baek, K.-H. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef] [PubMed]
- Stanic, Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge-A Review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Rong, L.; Ji, X.; Shen, M.; Chen, X.; Qi, X.; Li, Y.; Xie, J. Characterization of gallic acid-Chinese yam starch biodegradable film incorporated with chitosan for potential use in pork preservation. Food Res. Int. 2023, 164, 112331. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ortega, I.; Garica-Almendarez, B.E.; Santos-Lopez, E.M.; Amaro-Reyes, A.; Eleazar Barboza-Corona, J.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef]
- GB 2707-2016; National Standards for Food Safety Fresh (Frozen) Livestock and Poultry Products. China Standards Press: Beijing, China, 2016.
- Liu, C.-L.; Yang, J.; Bai, X.-H.; Cao, Z.-K.; Yang, C.; Ramakrishna, S.; Yang, D.-P.; Zhang, J.; Long, Y.-Z. Dual Antibacterial Effect of In Situ Electrospun Curcumin Composite Nanofibers to Sterilize Drug-Resistant Bacteria. Nanoscale Res. Lett. 2021, 16, 54. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Chen, Y.; Ding, H.; Wu, H.; Gao, Y.; Huang, S.; Wu, H.; Kong, D.; Yang, Z.; et al. Active and smart biomass film containing cinnamon oil and curcumin for meat preservation and freshness indicator. Food Hydrocoll. 2022, 133, 107979. [Google Scholar] [CrossRef]









| Film Type | Rq (nm) | Ra (nm) |
|---|---|---|
| Cur/ATH10:0 | 52.75 ± 6.58 a | 41.90 ± 7.21 a |
| Cur/ATH0:10 | 16.70 ± 0.57 c | 13.15 ± 0.64 c |
| Cur/ATH2:8 | 28.85 ± 2.05 b | 21.65 ± 0.92 bc |
| Cur/ATH5:5 | 30.50 ± 2.12 b | 21.90 ± 0.57 bc |
| Cur/ATH8:2 | 34.95 ± 4.17 b | 25.55 ± 5.73 b |
| Film Type | Thickness (mm) | WC (%) | WS (%) | WVP (10−10 g∙m/m2∙s∙Pa) | ROT (10−3 g/m2∙s) |
|---|---|---|---|---|---|
| Cur/ATH10:0 | 0.030 ± 0.001 a | 8.79 ± 2.66 b | 49.12 ± 3.74 c | 2.22 ± 0.25 bc | 2.96 ± 0.09 a |
| Cur/ATH0:10 | 0.026 ± 0.002 c | 20.82 ± 2.43 a | 63.58 ± 2.31 a | 2.67 ± 0.19 a | 2.81 ± 0.29 ab |
| Cur/ATH2:8 | 0.027 ± 0.002 bc | 17.32 ± 2.65 a | 58.00 ± 1.27 ab | 2.57 ± 0.22 ab | 2.75 ± 0.07 ab |
| Cur/ATH5:5 | 0.028 ± 0.001 ab | 16.88 ± 1.63 a | 56.27 ± 0.90 b | 2.37 ± 0.04 abc | 2.44 ± 0.36 b |
| Cur/ATH8:2 | 0.028 ± 0.002 ab | 14.31 ± 2.74 ab | 52.91 ± 1.60 bc | 2.09 ± 0.22 c | 2.87 ± 0.14 ab |
| Film Type | TS (Mpa) | EAB (%) |
|---|---|---|
| Cur/ATH10:0 | 45.03 ± 0.36 e | 13.25 ± 0.69 d |
| Cur/ATH0:10 | 52.71 ± 2.64 c | 15.30 ± 0.39 bc |
| Cur/ATH2:8 | 57.01 ± 0.72 b | 16.70 ± 0.32 ab |
| Cur/ATH5:5 | 62.96 ± 1.61 a | 17.42 ± 0.73 a |
| Cur/ATH8:2 | 49.06 ± 1.49 d | 14.73 ± 0.75 c |
| Film Type | L* | a* | b* | ΔE* |
|---|---|---|---|---|
| Cur/ATH10:0 | 85.30 ± 0.09 a | 9.61 ± 0.13 a | 61.98 ± 0.44 a | 60.92 ± 0.47 a |
| Cur/ATH0:10 | 85.51 ± 0.76 a | 5.05 ± 0.22 b | 4.47 ± 0.26 e | 11.15 ± 0.80 e |
| Cur/ATH2:8 | 85.26 ± 0.29 a | 0.39 ± 0.20 e | 48.37 ± 0.63 d | 46.72 ± 0.67 d |
| Cur/ATH5:5 | 84.79 ± 0.04 a | 1.88 ± 0.07 d | 57.52 ± 0.26 c | 55.83 ± 0.27 c |
| Cur/ATH8:2 | 85.42 ± 0.33 a | 3.28 ± 0.22 c | 59.48 ± 0.39 b | 57.72 ± 0.39 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Li, N.; Peng, H.; Yang, X.; Lin, D. The Development of Highly pH-Sensitive Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films Loaded with Anthocyanin/Curcumin for the Fresh-Keeping and Freshness Detection of Fresh Pork. Foods 2023, 12, 3719. https://doi.org/10.3390/foods12203719
Zhou S, Li N, Peng H, Yang X, Lin D. The Development of Highly pH-Sensitive Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films Loaded with Anthocyanin/Curcumin for the Fresh-Keeping and Freshness Detection of Fresh Pork. Foods. 2023; 12(20):3719. https://doi.org/10.3390/foods12203719
Chicago/Turabian StyleZhou, Siyu, Nan Li, Haonan Peng, Xingbin Yang, and Dehui Lin. 2023. "The Development of Highly pH-Sensitive Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films Loaded with Anthocyanin/Curcumin for the Fresh-Keeping and Freshness Detection of Fresh Pork" Foods 12, no. 20: 3719. https://doi.org/10.3390/foods12203719
APA StyleZhou, S., Li, N., Peng, H., Yang, X., & Lin, D. (2023). The Development of Highly pH-Sensitive Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films Loaded with Anthocyanin/Curcumin for the Fresh-Keeping and Freshness Detection of Fresh Pork. Foods, 12(20), 3719. https://doi.org/10.3390/foods12203719