The Preparation and Properties of Amino-Carboxymethyl Chitosan-Based Antibacterial Hydrogel Loaded with ε-Polylysine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
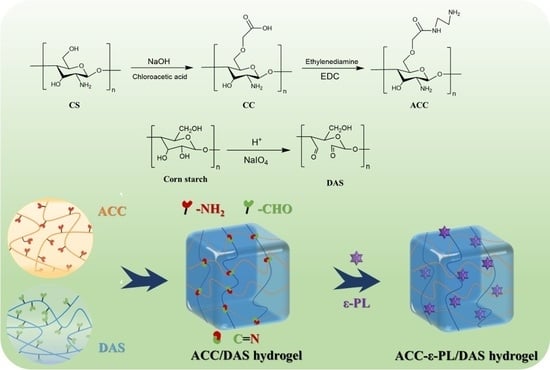
2.2. Synthesis of ACC and DAS
2.3. Determination of Amino Content of ACC and Aldehyde Group Content of DAS
2.4. Preparation of ACC/DAS Hydrogels
2.5. Characterizations of ACC, DAS, and ACC/DAS Hydrogels
2.6. Swelling Degree of ACC/DAS Hydrogels
2.7. Loading Efficiency of ACC/DAS Hydrogels
2.8. Antibacterial Activity of ACC/DAS Hydrogels
2.9. Statistical Analysis
3. Results
3.1. Characterizations of ACC and DAS
3.2. Preparation and Characterizations of ACC/DAS Hydrogels
3.3. Swelling Behavior of the Hydrogels
3.4. Loading Efficiency of the Hydrogels
3.5. Antibacterial Activity of the Hydrogels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Zhao, Y.; Wu, N.; Chen, S.; Xu, M.; Du, H.; Yao, Y.; Tu, Y. Egg white protein-based delivery system for bioactive substances: A review. Crit. Rev. Food Sci. Nutr. 2022, 43, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Li, M.F.; Peng, F.; Zhong, S.R.; Huang, Z.; Zong, M.H.; Lou, W.Y. Oxidized high-amylose starch macrogel as a novel delivery vehicle for probiotic and bioactive substances. Food Hydrocolloid 2021, 114, 106578. [Google Scholar] [CrossRef]
- Shima, S.; Sakai, H. Polylysine produced by Streptomyces. Agric. Biol. Chem. 1977, 41, 1807–1809. [Google Scholar] [CrossRef]
- Zahi, M.R.; Hattab, M.; Liang, H.; Yuan, Q. Enhancing the antimicrobial activity of d-limonene nanoemulsion with the inclusion of ε-polylysine. Food Chem. 2017, 221, 18–23. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Interaction of cationic antimicrobial (ε-polylysine) with food-grade biopolymers: Dextran, chitosan, carrageenan, alginate, and pectin. Food Res. Int. 2014, 64, 396–401. [Google Scholar] [CrossRef]
- Song, M.; Lopez-Pena, C.L.; McClements, D.J.; Decker, E.A.; Xiao, H. Safety evaluation and lipid-lowering effects of food-grade biopolymer complexes (ε-polylysine-pectin) in mice fed a high-fat diet. Food Funct. 2017, 8, 1822–1829. [Google Scholar] [CrossRef]
- Saeedi, M.; Vahidi, O.; Moghbeli, M.R.; Ahmadi, S.; Asadnia, M.; Akhavan, O.; Seidi, F.; Rabiee, M.; Saeb, M.R.; Webster, T.J.; et al. Customizing nano-chitosan for sustainable drug delivery. J. Control. Release 2022, 350, 175–192. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; De la Flor, S.; Kozlowska, J. From Supramolecular Hydrogels to Multifunctional Carriers for Biologically Active Substances. Nano Micro Mater. Healthc. 2021, 22, 7402. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Sawsan, D.; Mohamed, H. Biocompatible hydrogel based on aldehyde-functionalized cellulose and chitosan for potential control drug release. Sustain. Chem. Pharm. 2021, 21, 100419. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, B.; Li, D.; Ren, J.; Zhu, Y.; Sun, Z.; Chen, Y. Semi-Unzipping of Chitosan-Sodium Alginate Polyelectrolyte Gel for Efficient Capture of Metallic Mineral Ions from Tannery Effluent. Chem. Eng. J. 2022, 452, 139532. [Google Scholar] [CrossRef]
- Pan, Q.; Zhou, C.; Yang, Z.; He, Z.; Wang, C.; Liu, Y.; Song, S.; Gu, H.; Hong, K.; Yu, L.; et al. Preparation and characterization of chitosan derivatives modified with quaternary ammonium salt and quaternary phosphate salt and its effect on tropical fruit preservation. Food Chem. 2022, 387, 132878. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohyd Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, M.; Yuan, R.; Yang, Y.; Lu, Z.; Ge, L. Biocompatible Self-Healing Coating Based on Schiff Base for Promoting Adhesion of Coral Cells. Acs Appl. Bio Mater. 2020, 3, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Dziadek, K.; Salagierski, S. Newly crosslinked chitosan- and chitosan-pectin-based hydrogels with high antioxidant and potential anticancer activity. Carbohyd Polym. 2022, 290, 119486. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Tian, C.; Zhang, G.L.; Hao, H.; Hou, H.M. Novel procyanidins-loaded chitosan-graft-polyvinyl alcohol film with sustained antibacterial activity for food packaging. Food Chem. 2021, 365, 130534. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Guan, S.; Dong, A.; Cao, Y.; Chen, C. A novel Ag/AgO/carboxymethyl chitosan bacteriostatic hydrogel for drug delivery. Mater. Res. Express 2020, 7, 085431. [Google Scholar] [CrossRef]
- Fan, S.; Li, Z.; Fan, C.; Chen, J.; Huang, H. Fast-thermoresponsive carboxylated carbon nanotube/chitosan aerogels with switchable wettability for oil/water separation. J. Hazard. Mater. 2022, 433, 128808. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Ali, A.; Yang, W.; Zhang, R.; Li, Y.; Zhang, L.; Li, J. Chitosan and carboxymethyl chitosan mimic biomineralization and promote microbially induced calcium precipitation. Carbohyd Polym. 2022, 287, 119335. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.X.; Mo, X.M.; Pan, J.F. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef]
- Olanipekun, E.O.; Ayodele, O.; Olatunde, O.C.; Olusegun, S.J. Comparative studies of chitosan and carboxymethyl chitosan doped with nickel and copper: Characterization and antibacterial potential. Int. J. Biol. Macromol. 2021, 183, 1971–1977. [Google Scholar] [CrossRef]
- Mondal, M.d.; Ibrahim, H.; Firoz, A. Cellulosic fibres modified by chitosan and synthesized ecofriendly carboxymethyl chitosan from prawn shell waste. J. Text. Inst. 2020, 111, 49–59. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, W.; Li, X.; Xu, Y.; Gao, H.; Zheng, B. The structure, prties, synthesis method and antimicrobial mechanism of ε-polylysine with the preservative effects for aquatic products. Trends Food Sci. Technol. 2023, 139, 104131. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, W.; Xiao, J.; Zhao, X.; Zhu, Y.; Wu, Y. Preparation and characterization of dialdehyde starch by one-step acid hydrolysis and oxidation. Int. J. Biol. Macromol. 2017, 103, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.H.; Han, Y.; Yue, F.H. The effects of pulsed electric fields treatment on the structure and physicochemical properties of dialdehyde starch. Food Chem. 2023, 408, 135231. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wan, Y.; Wang, L.; Zhou, T. Preparation, characterization and releasing property of antibacterial nano-capsules composed of ε-PL-EGCG and sodium alginate-chitosan. Int. J. Biol. Macromol. 2022, 204, 652–660. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Zhou, Y.; Wu, Y.; Ouyang, J. Influence of ultrasound and microwave treatments on the structural and thermal properties of normal maize starch and potato starch: A comparative study. Food Chem. 2022, 377, 131990. [Google Scholar] [CrossRef]
- Ren, J.; Li, M.; Yuan, R.; Pang, A.; Lu, Z.; Ge, L. Adherent self-healing chitosan/dialdehyde starch coating. Mat. Sci. Eng. 2020, 586, 124203. [Google Scholar] [CrossRef]
- Raveendran, R.L.; Anirudhan, T.S. Development of macroscopically ordered liquid crystalline hydrogels from biopolymers with robust antibacterial activity for controlled drug delivery applications. Polym. Chem. 2021, 12, 3992–4005. [Google Scholar] [CrossRef]
- Vlasceanu, G.M.; Crica, L.E.; Pandele, A.M.; Ionita, M. Graphene Oxide Reinforcing Genipin Crosslinked Chitosan-Gelatin Blend Films. Coatings 2020, 10, 189. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Rueda-Mira, S.; Felipe Santa Marín, J.; Álvarez-López, C. Development of a novel silk sericin-based hydrogel film by mixture design. J. Polym. Res. 2023, 30, 120. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Zhou, C.; Qiao, C.; Yuan, F.; Liu, Q.; Luo, X. Preparation and Properties of Composite Hydrogels Based on Microgels Containing Chitosan. J. Macromol. Sci. B 2022, 61, 557–570. [Google Scholar] [CrossRef]
- Taghreed, H.A.; Abir, S.; Ghada, B.; David, R.; Nadia, G. Fabrication of sustainable hydrogels-based chitosan Schiff base and their potential applications. Arab. J. Chem. 2022, 15, 103511. [Google Scholar]
- Jeong, J.P.; Kim, K.; Kim, J.; Kim, Y.; Jung, S. New Polyvinyl Alcohol/Succinoglycan-Based Hydrogels for pH-Responsive Drug Delivery. Polymers 2023, 15, 3009. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, N. Gelatin/carboxymethyl cellulose based stimuli-responsive hydrogels for controlled delivery of 5-fluorouracil, development, in vitro characterization, in vivo safety and bioavailability evaluation. Carbohyd Polym. 2021, 257, 117617. [Google Scholar] [CrossRef] [PubMed]
- Kopka, B.; Kost, B.; Rajkowska, K. A simple strategy for efficient preparation of networks based on poly(2-isopropenyl-2-oxazoline), poly(ethylene oxide), and selected biologically active compounds: Novel hydrogels with antibacterial properties. Soft Matter 2021, 17, 10683–10695. [Google Scholar] [CrossRef] [PubMed]








| Samples | Tensile Strength (MPa) | Breaking Elongation (%) | Elastic Modulus (MPa) |
|---|---|---|---|
| ACC1/DAS | 43.3 ± 7.4 a | 40.5 ± 0.03 a | 81.47 ± 5.4 a |
| ACC2/DAS | 43.7 ± 5.7 a | 10.2 ± 0.04 b | 586.2 ± 3.7 b |
| ACC3/DAS | 59.3 ± 7.6 b | 7.6 ± 0.04 b | 663.7 ± 1.9 b |
| ACC4/DAS | 63.2 ± 8.4 c | 6.4 ± 0.01 b | 741.1 ± 2.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qiu, Y.; Hou, H.; Zhang, G.; Hao, H.; Bi, J. The Preparation and Properties of Amino-Carboxymethyl Chitosan-Based Antibacterial Hydrogel Loaded with ε-Polylysine. Foods 2023, 12, 3807. https://doi.org/10.3390/foods12203807
Li Y, Qiu Y, Hou H, Zhang G, Hao H, Bi J. The Preparation and Properties of Amino-Carboxymethyl Chitosan-Based Antibacterial Hydrogel Loaded with ε-Polylysine. Foods. 2023; 12(20):3807. https://doi.org/10.3390/foods12203807
Chicago/Turabian StyleLi, Yixi, Yulong Qiu, Hongman Hou, Gongliang Zhang, Hongshun Hao, and Jingran Bi. 2023. "The Preparation and Properties of Amino-Carboxymethyl Chitosan-Based Antibacterial Hydrogel Loaded with ε-Polylysine" Foods 12, no. 20: 3807. https://doi.org/10.3390/foods12203807
APA StyleLi, Y., Qiu, Y., Hou, H., Zhang, G., Hao, H., & Bi, J. (2023). The Preparation and Properties of Amino-Carboxymethyl Chitosan-Based Antibacterial Hydrogel Loaded with ε-Polylysine. Foods, 12(20), 3807. https://doi.org/10.3390/foods12203807