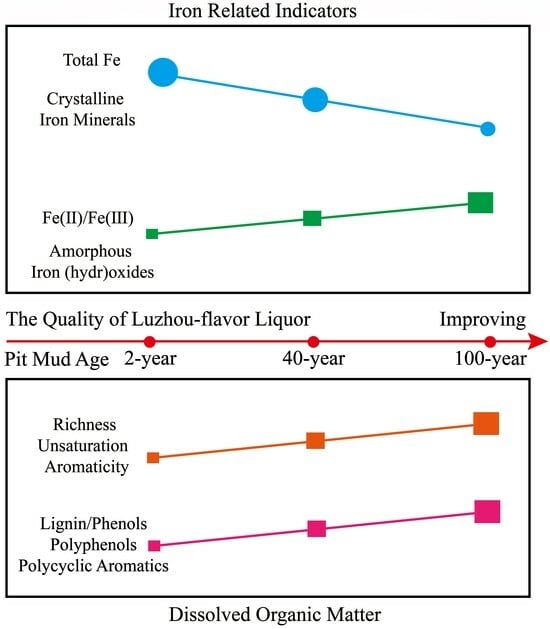
Difference Analysis of the Composition of Iron (Hydr)Oxides and Dissolved Organic Matter in Pit Mud of Different Pit Ages in Luzhou Laojiao and Its Implications for the Ripening Process of Pit Mud
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Environmental Description
2.2. Determination and Characterization of Physicochemical Properties of Samples
2.3. Extraction and Determination of DOM in Samples
2.3.1. DOM Extraction
2.3.2. FT-ICR-MS Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Mineral Composition and Physicochemical Properties of Different Samples
3.2. Changes in DOM Molecular Composition during Aging of Pit Mud
3.3. Association of DOM Molecules with Iron (Hydr)Oxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, J.; Chen, L.; Yang, S.; Li, Y.; Jin, L.; He, X.; He, L.; Ao, X.; Liu, S.; Liu, A.; et al. Metagenome and analysis of metabolic potential of the microbial community in pit mud used for Chinese strong-flavor liquor production. Food Res. Int. 2021, 143, 110294. [Google Scholar] [CrossRef]
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food. Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Chai, L.J.; Qian, W.; Zhong, X.Z.; Zhang, X.J.; Lu, Z.M.; Zhang, S.Y.; Wang, S.T.; Shen, C.H.; Shi, J.S.; Xu, Z.H. Mining the Factors Driving the Evolution of the Pit Mud Microbiome under the Impact of Long-Term Production of Strong-Flavor Baijiu. Appl. Environ. Microbiol. 2021, 87, e88521. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, B.; Fan, G.; Teng, C.; Xiong, K.; Zhu, Y.; Li, J.; Li, X. The brewing process and microbial diversity of strong flavour Chinese spirits: A review. J. Inst. Brew. 2017, 123, 5–12. [Google Scholar] [CrossRef]
- Guan, T.; Lin, Y.; Chen, K.; Ou, M.; Zhang, J. Physicochemical Factors Affecting Microbiota Dynamics During Traditional Solid-State Fermentation of Chinese Strong-Flavor Baijiu. Front. Microbiol. 2020, 11, 2090. [Google Scholar] [CrossRef]
- Liu, M.; Tang, Y.; Zhao, K.; Liu, Y.; Guo, X.; Ren, D.; Yao, W.; Tian, X.; Gu, Y.; Yi, B.; et al. Determination of the fungal community of pit mud in fermentation cellars for Chinese strong-flavor liquor, using DGGE and Illumina MiSeq sequencing. Food Res. Int. 2017, 91, 80–87. [Google Scholar] [CrossRef]
- Liu, M.; Tang, Y.; Guo, X.; Zhao, K.; Tian, X.; Liu, Y.; Yao, W.; Deng, B.; Ren, D.; Zhang, X. Deep sequencing reveals high bacterial diversity and phylogenetic novelty in pit mud from Luzhou Laojiao cellars for Chinese strong-flavor Baijiu. Food Res. Int. 2017, 102, 68–76. [Google Scholar]
- Zhang, H.; Meng, Y.; Wang, Y.; Zhou, Q.; Li, A.; Liu, G.; Li, J.; Xing, X. Prokaryotic communities in multidimensional bottom-pit-mud from old and young pits used for the production of Chinese Strong-Flavor Baijiu. Food Chem. 2020, 312, 126084. [Google Scholar]
- Chai, L.; Xu, P.; Qian, W.; Zhang, X.; Ma, J.; Lu, Z.; Wang, S.; Shen, C.; Shi, J.; Xu, Z. Profiling the Clostridia with butyrate-producing potential in the mud of Chinese liquor fermentation cellar. Int. J. Food Microbiol. 2019, 297, 41–50. [Google Scholar]
- Hu, X.; Du, H.; Ren, C.; Xu, Y. Illuminating Anaerobic Microbial Community and Cooccurrence Patterns across a Quality Gradient in Chinese Liquor Fermentation Pit Muds. Appl. Environ. Microbiol. 2016, 82, 2506–2515. [Google Scholar] [CrossRef]
- Bahureksa, W.; Tfaily, M.M.; Boiteau, R.M.; Young, R.B.; Logan, M.N.; McKenna, A.M.; Borch, T. Soil Organic Matter Characterization by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR MS): A Critical Review of Sample Preparation, Analysis, and Data Interpretation. Environ. Sci. Technol. 2021, 55, 9637–9656. [Google Scholar] [CrossRef]
- Han, H.; Feng, Y.; Chen, J.; Xie, Q.; Chen, S.; Sheng, M.; Zhong, S.; Wei, W.; Su, S.; Fu, P. Acidification impacts on the molecular composition of dissolved organic matter revealed by FT-ICR MS. Sci. Total Environ. 2022, 805, 150284. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V.; Liu, Q. Magnetic enhancement is linked to and precedes hematite formation in aerobic soil. Geophys. Res. Lett. 2006, 33, 2. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N.; Kirillova, N.P. Application of the CIE-L*a*b* System to Characterize Soil Color. Soil Phys. 2016, 49, 1337–1346. [Google Scholar] [CrossRef]
- NY/T 1121.6-2006; Soil Testing-Part 6: Method for Determination of Soil Organic Matter. Ministry of Agriculture of the China: Beijing, China, 2006.
- NY/T 1121.7-2014; Soil Testing-Part 7: Method for Determination of Available Phosphorus in Soil. Ministry of Agriculture of the China: Beijing, China, 2014.
- Lu, M.; Wang, X.; Li, Y.; Liu, H.; An, X.; Lian, B. Soil microbial community structure and environmental effects of serpentine weathering under different vegetative covers in the serpentine mining area of Donghai County, China. Sci. Total Environ. 2022, 835, 155452. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Xue, J.; Deng, Y.; Luo, Y.; Du, Y.; Yang, Y.; Cheng, Y.; Xie, X.; Gan, Y.; Wang, Y. Unraveling the impact of iron oxides-organic matter complexes on iodine mobilization in alluvial-lacustrine aquifers from central Yangtze River Basin. Sci. Total Environ. 2022, 814, 151930. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Chen, J.; Castellano, M.J.; Ye, C.; Zhang, N.; Miao, Y.; Zheng, H.; Li, J.; Ding, W. Oxygen availability regulates the quality of soil dissolved organic matter by mediating microbial metabolism and iron oxidation. Glob. Chang. Biol. 2022, 28, 7410–7427. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Ohno, T.; Parr, T.B.; Gruselle, M.C.I.; Fernandez, I.J.; Sleighter, R.L.; Hatcher, P.G. Molecular Composition and Biodegradability of Soil Organic Matter: A Case Study Comparing Two New England Forest Types. Environ. Sci. Technol. 2014, 48, 7229–7236. [Google Scholar] [CrossRef]
- Rezapour, S.; Azhah, H.; Momtaz, H.R.; Ghaemian, N. Changes in forms and distribution pattern of soil iron oxides due to long-term cropping in the Northwest of Iran. Environ. Earth Sci. 2015, 73, 7275–7286. [Google Scholar] [CrossRef]
- Li, X.; Sun, G.; Chen, S.; Fang, Z.; Yuan, H.; Shi, Q.; Zhu, Y. Molecular Chemodiversity of Dissolved Organic Matter in Paddy Soils. Environ. Sci. Technol. 2018, 52, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.P.; Dittmar, T. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 2006, 20, 926–932. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, C.; He, C.; Li, P.; Wang, Y.; Pang, Y.; Shi, Q.; He, D. Characterization of dissolved organic matter processing between surface sediment porewater and overlying bottom water in the Yangtze River Estuary. Water Res. 2022, 215, 118260. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Q.; Hu, X.; Zeng, H.; Deng, P.; He, C.; Shi, Q. Lake Chemodiversity Driven by Natural and Anthropogenic Factors. Environ. Sci. Technol. 2022, 56, 5910–5919. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Li, X.; Wang, J. Deciphering the Fingerprint of Dissolved Organic Matter in the Soil Amended with Biodegradable and Conventional Microplastics Based on Optical and Molecular Signatures. Environ. Sci. Technol. 2022, 56, 15746–15759. [Google Scholar] [CrossRef]
- Torrent, J.; Liu, Q.; Bloemendal, J.; Barron, V. Magnetic enhancement and iron oxides in the upper Luochuan loess-paleosol sequence, Chinese Loess Plateau. Soil Sci. Soc. Am. J. 2007, 71, 1570–1578. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yuan, Y.J.; Liao, Z.M.; Zhang, W.X. Use of microbial indicators combined with environmental factors coupled with metrology tools for discrimination and classification of Luzhou-flavoured pit muds. J. Appl. Microbiol. 2017, 123, 933–943. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, Z.; Zhang, W. Effect of Pit Mud on Bacterial Community and Aroma Components in Yellow Water and Their Changes during the Fermentation of Chinese Strong-Flavor Liquor. Foods 2020, 9, 372. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xue, X.; Xie, X.; Siebecker, M.G.; Sparks, D.L.; Wang, Y. Mechanistic insights into iodine enrichment in groundwater during the transformation of iron minerals in aquifer sediments. Sci. Total Environ. 2020, 745, 140922. [Google Scholar] [CrossRef]
- Frommer, J.; Voegelin, A.; Dittmar, J.; Marcus, M.A.; Kretzschmar, R. Biogeochemical processes and arsenic enrichment around rice roots in paddy soil: Results from micro-focused X-ray spectroscopy. Eur. J. Soil Sci. 2011, 62, 305–317. [Google Scholar] [CrossRef]
- Duan, X.; Li, Z.; Li, Y.; Yuan, H.; Gao, W.; Chen, X.; Ge, T.; Wu, J.; Zhu, Z. Iron–organic carbon associations stimulate carbon accumulation in paddy soils by decreasing soil organic carbon priming. Soil Biol. Biochem. 2023, 179, 108972. [Google Scholar] [CrossRef]
- Zhao, Y.; Moore, O.W.; Xiao, K.; Curti, L.; Fariña, A.O.; Banwart, S.A.; Peacock, C.L. The role and fate of organic carbon during aging of ferrihydrite. Geochim. Cosmochim. Acta 2022, 335, 339–355. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Kleber, M.; Fendorf, S. Are oxygen limitations under recognized regulators of organic carbon turnover in upland soils? Biogeochemistry 2016, 127, 157–171. [Google Scholar] [CrossRef]
- Wu, S.; You, F.; Boughton, B.; Liu, Y.; Nguyen, T.A.H.; Wykes, J.; Southam, G.; Robertson, L.M.; Chan, T.; Lu, Y.; et al. Chemodiversity of Dissolved Organic Matter and Its Molecular Changes Driven by Rhizosphere Activities in Fe Ore Tailings Undergoing Eco-Engineered Pedogenesis. Environ. Sci. Technol. 2021, 55, 13045–13060. [Google Scholar] [CrossRef]
- Chapman, S.J.; Campbell, C.D.; Fraser, A.R.; Puri, G. FTIR spectroscopy of peat in and bordering Scots pine woodland; relationship with chemical and biological properties. Soil Biol. Biochem. 2001, 33, 1193–1200. [Google Scholar] [CrossRef]
- Artz, R.R.E.; Chapman, S.J.; Jean Robertson, A.H.; Potts, J.M.; Laggoun-Défarge, F.; Gogo, S.; Comont, L.; Disnar, J.; Francez, A. FTIR spectroscopy can be used as a screening tool for organic matter quality in regenerating cutover peatlands. Soil Biol. Biochem. 2008, 40, 515–527. [Google Scholar] [CrossRef]
- Li, Y.; Heal, K.; Wang, S.; Cao, S.; Zhou, C. Chemodiversity of Soil Dissolved Organic Matter and Its Association with Soil Microbial Communities Along a Chronosequence of Chinese Fir Monoculture Plantations. Front. Microbiol. 2021, 12, 729344. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Q.; Liao, L.; Duan, Z.; Liu, S.; Chen, L.; Zhu, Q.; Zhong, A. Hydrological isolation affected the chemo-diversity of dissolved organic matter in a large river-connected lake (Poyang Lake, China). Sci. Total Environ. 2022, 851, 158047. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Z.; Liu, L.; Qi, K.; Xie, X. Novel insights into the temporal molecular fractionation of dissolved black carbon at the iron oxyhydroxide-water interface. Water Res. 2023, 229, 119410. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.; Wang, S.; Luo, L.; Cao, D.; Christie, P. Molecular-Scale Investigation with ESI-FT-ICR-MS on Fractionation of Dissolved Organic Matter Induced by Adsorption on Iron Oxyhydroxides. Environ. Sci. Technol. 2016, 50, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Bolan, N.S.; Lai, J.; Wang, Y.; Jin, X.; Kirkham, M.B.; Wu, X.; Fang, Z.; Zhang, Y.; Wang, H. Interactions between organic matter and Fe (hydr)oxides and their influences on immobilization and remobilization of metal(loid)s: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4016–4037. [Google Scholar] [CrossRef]
- Huang, Z.; Lv, J.; Cao, D.; Zhang, S. Iron plays an important role in molecular fractionation of dissolved organic matter at soil-water interface. Sci. Total Environ. 2019, 670, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Burton, E.D.; Johnston, S.G.; Hockmann, K.; Choppala, G. Humic acid impacts antimony partitioning and speciation during iron(II)-induced ferrihydrite transformation. Sci. Total Environ. 2019, 683, 399–410. [Google Scholar] [CrossRef]





| Parameter | CK | 2-PM | 40-PM | 100-PM |
|---|---|---|---|---|
| pH | 6.16 ± 0.04 b | 4.04 ± 0.03 d | 5.10 ± 0.22 c | 6.62 ± 0.11 a |
| MC (%) | 7.57 ± 0.33 d | 23.71 ± 1.53 c | 32.60 ± 3.28 b | 43.87 ± 1.68 a |
| OM (g/kg) | 22.62 ± 1.74 d | 89.14 ± 4.72 c | 168.99 ± 26.21 b | 223.78 ± 6.95 a |
| AP (g/kg) | 0.05 ± 0.00 c | 0.30 ± 0.02 c | 10.54 ± 0.40 b | 13.20 ± 0.36 a |
| DOC (mg/L) | 66.84 ± 3.38 d | 381.58 ± 18.07 c | 828.23 ± 91.26 b | 1202.88 ± 78.97 a |
| Carbonate (%) | 3.55 ± 0.28 a | 1.41 ± 0.13 c | 2.33 ± 0.11 b | 2.71 ± 0.30 b |
| TFe (g/kg) | 31.99 ± 0.50 a | 24.26 ± 1.55 b | 20.21 ± 0.18 c | 15.24 ± 0.28 d |
| Fe(II) (g/kg) | 0.45 ± 0.03 d | 0.74 ± 0.05 c | 2.04 ± 0.07 b | 3.79 ± 0.13 a |
| Fe(III) (g/kg) | 31.54 ± 0.53 a | 23.52 ± 1.53 b | 18.17 ± 0.19 c | 11.44 ± 0.33 d |
| Fe(II)/Fe(III) | 0.01 ± 0.00 c | 0.03 ± 0.01 c | 0.11 ± 0.01 b | 0.33 ± 0.02 a |
| Fed (g/kg) | 12.44 ± 0.22 a | 10.52 ± 0.32 b | 9.20 ± 0.25 c | 8.83 ± 0.05 c |
| Feo (g/kg) | 1.51 ± 0.22 d | 3.69 ± 0.04 c | 6.16 ± 0.07 b | 7.54 ± 0.51 a |
| Fec (g/kg) | 10.93 ± 0.31 a | 6.83 ± 0.29 b | 3.03 ± 0.27 c | 1.29 ± 0.51 d |
| Sample | Formula Number | O/C | H/C | DBE | AI | NOSC | CRAMs |
|---|---|---|---|---|---|---|---|
| CK | 3636 ± 227 c | 0.22 ± 0.01 c | 1.94 ± 0.08 a | 3.20 ± 0.13 c | 0.05 ± 0.03 c | −1.44 ± 0.09 d | 572 ± 79 c |
| 2-PM | 5459 ± 901 b | 0.25 ± 0.01 bc | 1.67 ± 0.04 b | 4.45 ± 0.14 b | 0.10 ± 0.01 b | −0.88 ± 0.04 c | 1419 ± 532 b |
| 40-PM | 6762 ± 294 ab | 0.27 ± 0.01 ab | 1.53 ± 0.02 bc | 5.38 ± 0.32 b | 0.13 ± 0.01 ab | −0.69 ± 0.01 b | 2514 ± 142 a |
| 100-PM | 7858 ± 224 a | 0.28 ± 0.16 a | 1.43 ± 0.11 c | 6.65 ± 0.87 a | 0.16 ± 0.01 a | −0.55 ± 0.01 a | 2616 ± 164 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, K.; Deng, B.; Song, P.; Ding, H.; Liu, H.; Lian, B. Difference Analysis of the Composition of Iron (Hydr)Oxides and Dissolved Organic Matter in Pit Mud of Different Pit Ages in Luzhou Laojiao and Its Implications for the Ripening Process of Pit Mud. Foods 2023, 12, 3962. https://doi.org/10.3390/foods12213962
Jiao K, Deng B, Song P, Ding H, Liu H, Lian B. Difference Analysis of the Composition of Iron (Hydr)Oxides and Dissolved Organic Matter in Pit Mud of Different Pit Ages in Luzhou Laojiao and Its Implications for the Ripening Process of Pit Mud. Foods. 2023; 12(21):3962. https://doi.org/10.3390/foods12213962
Chicago/Turabian StyleJiao, Kairui, Bo Deng, Ping Song, Hailong Ding, Hailong Liu, and Bin Lian. 2023. "Difference Analysis of the Composition of Iron (Hydr)Oxides and Dissolved Organic Matter in Pit Mud of Different Pit Ages in Luzhou Laojiao and Its Implications for the Ripening Process of Pit Mud" Foods 12, no. 21: 3962. https://doi.org/10.3390/foods12213962
APA StyleJiao, K., Deng, B., Song, P., Ding, H., Liu, H., & Lian, B. (2023). Difference Analysis of the Composition of Iron (Hydr)Oxides and Dissolved Organic Matter in Pit Mud of Different Pit Ages in Luzhou Laojiao and Its Implications for the Ripening Process of Pit Mud. Foods, 12(21), 3962. https://doi.org/10.3390/foods12213962