Production of Kudzu Starch Gels with Superior Mechanical and Rheological Properties through Submerged Ethanol Exposure and Implications for In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
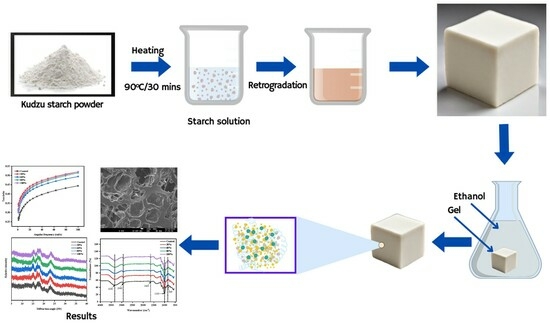
2.2. Kudzu Starch Gel Formation
2.3. Gel Syneresis, Size, and Color Characteristics
2.4. Mechanical Properties
2.5. Rheological Properties
2.6. Scanning Electron Microscopy (SEM)
2.7. X-ray Diffraction (XRD)
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.9. Differential Scanning Calorimetry (DSC)
2.10. In Vitro Digestibility
2.11. Statistical Analysis
3. Results and Discussion
3.1. The Textural Characteristics of Kudzu Starch Gels
3.2. Thermal Properties of the Kudzu Gels
3.3. Gel Syneresis, Size, and Colorimetric Properties
3.4. Rheological Properties
3.5. Scanning Electron Microscopy
3.6. X-ray Diffraction Analysis
3.7. FTIR Spectra Analysis
3.8. In Vitro Digestion Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, P.; Li, Y.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q. Rapid production of corn starch gels with high mechanical properties through alcohol soaking. Int. J. Biol. Macromol. 2020, 163, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Nepovinnykh, N.V.; Kliukina, O.N.; Ptichkina, N.M.; Bostan, A. Hydrogel based dessert of low calorie content. Food Hydrocoll. 2019, 86, 184–192. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Liu, F.; Wu, D.; Hong, W. Mechanism study on mechanical properties of physical–chemical hybrid hydrogels by coarse-grained molecular dynamics simulations. ACS Appl. Polym. Mater. 2023, 5, 1707–1714. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, Z.; Liang, X.; Zhou, Y.; Zhao, G. Insights into network rearrangement of konjac glucomannan gel induced by post-gelation soaking. Food Hydrocoll. 2023, 145, 109044. [Google Scholar] [CrossRef]
- Sun, Y.; Li, F.; Luan, Y.; Li, P.; Dong, X.; Chen, M.; Dai, L.; Sun, Q. Food hydrocolloids gelatinization, pasting, and rheological properties of pea starch in alcohol solution. Food Hydrocoll. 2021, 112, 106331. [Google Scholar] [CrossRef]
- Arabi, S.H.; Haselberger, D.; Hinderberger, D. The effect of ethanol on gelation, nanoscopic, and macroscopic properties of serum albumin hydrogels. Molecules 2020, 25, 1927. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.K.; Luan, F.; Xu, B. Morphology, crystallinity, pasting, thermal and quality characteristics of starches from adzuki bean (Vigna angularis L.) and edible kudzu (Pueraria thomsonii Benth). Int. J. Biol. Macromol. 2017, 105, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, B.; Janaswamy, S.; Guo, L. Structural and functional modifications of kudzu starch modified by branching enzyme. Int. J. Food Prop. 2019, 22, 952–966. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Fang, Y. Structure, properties and applications of kudzu starch. Food Hydrocoll. 2021, 119, 106817. [Google Scholar] [CrossRef]
- Tovar, J.; Melito, C.; Herrera, E.; Rascón, A.; Pérez, E. Resistant starch formation does not parallel syneresis tendency in different starch gels. Food Chem. 2002, 76, 455–459. [Google Scholar] [CrossRef]
- Ekumah, J.-N.; Ma, Y.; Akpabli-Tsigbe, N.D.; Kwaw, E.; Jie, H.; Quaisie, J.; Manqing, X.; Johnson Nkuma, N.A. Effect of selenium supplementation on yeast growth, fermentation efficiency, phytochemical and antioxidant activities of mulberry wine. LWT 2021, 146, 111425. [Google Scholar] [CrossRef]
- Adewale, F.J.; Lucky, A.P.; Oluwabunmi, A.P.; Boluwaji, E.F. Selecting the most appropriate model for rheological characterization of synthetic based drilling mud. Int. J. Appl. Eng. Res. 2017, 12, 7614–7629. [Google Scholar]
- Ahmad, M.; Mudgil, P.; Gani, A.; Hamed, F.; Masoodi, F.A.; Maqsood, S. Nano-encapsulation of catechin in starch nanoparticles: Characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019, 270, 95–104. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, P.; Fan, L.; Sun, Y. Effects of ultrasound treatment on the starch properties and oil absorption of potato chips. Ultrason. Sonochem. 2021, 70, 105347. [Google Scholar] [CrossRef]
- Wu, J.; Xu, S.; Huang, Y.; Zhang, X.; Liu, Y.; Wang, H.; Zhong, Y.; Bai, L.; Liu, C. Prevents kudzu starch from agglomeration during rapid pasting with hot water by a non-destructive superheated steam treatment. Food Chem. 2022, 386, 132819. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Yang, Y.; Liu, K. Mechanical characterisation of hydrogels for tissue engineering applications. Top. Tissue Eng. 2008, 4, 1–16. [Google Scholar]
- Alam, F.; Hasnain, A. Studies on swelling and solubility of modified starch from taro (Colocasia esculenta): Effect of pH and temperature. Agric. Conspec. Sci. 2009, 74, 45–50. [Google Scholar]
- Mohamed, A.; Hussain, S.; Alamri, M.S.; Ibraheem, M.A.; Abdo Qasem, A.A.; Ababtain, I.A. Physicochemical Properties of Starch Binary Mixtures with Cordia and Ziziphus Gums. Processes 2022, 10, 180. [Google Scholar] [CrossRef]
- Akonor, P.T.; Tortoe, C.; Oduro-Yeboah, C.; Saka, E.A.; Ewool, J. Physicochemical, microstructural, and rheological characterization of tigernut (Cyperus esculentus) Starch. Int. J. Food Sci. 2019, 2019, 3830651. [Google Scholar] [CrossRef]
- Mohamed, I.O. Effects of processing and additives on starch physicochemical and digestibility properties. Carbohydr. Polym. Technol. Appl. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Mohd Shukri, A.; Cheng, L.H. The Properties of Different Starches under the Influence of Glucono-Delta-Lactone at Different Concentrations. Foods 2023, 12, 1770. [Google Scholar] [CrossRef]
- Song, Q.; Wu, L.; Li, S.; Zhao, G.; Cheng, Y.; Zhou, Y. Aggregation of konjac glucomannan by ethanol under low-alkali treatment. Food Chem. X 2022, 15, 100407. [Google Scholar] [CrossRef]
- Roux, D.C.D.; Jeacomine, I.; Maîtrejean, G.; Caton, F.; Rinaudo, M. Characterization of Agarose Gels in Solvent and Non-Solvent Media. Polymers 2023, 15, 2162. [Google Scholar] [CrossRef]
- Cassanelli, M.; Norton, I.; Mills, T. Effect of alcohols on gellan gum gel structure: Bridging the molecular level and the three-dimensional network. Food Struct. 2017, 14, 112–120. [Google Scholar] [CrossRef]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the road to biopolymer aerogels—Dealing with the solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Landim Parente, G.D.; de Almeida Macêdo, F.; Melo Diniz, N.C.; da Conceição, M.M.; Ubbink, J.; Mattos Braga, A.L. Observations on the formation and textural properties of “tapiocas”, a traditional cassava-based food from the Northeast of Brazil. Int. J. Gastron. Food Sci. 2021, 26, 100417. [Google Scholar] [CrossRef]
- Aleixandre, A.; Benavent-Gil, Y.; Moreira, R.; Rosell, C.M. Food hydrocolloids in vitro digestibility of gels from different starches: Relationship between kinetic parameters and microstructure. Food Hydrocoll. 2021, 120, 106909. [Google Scholar] [CrossRef]
- Benavent-Gil, Y.; Rosell, C.M. Comparison of porous starches obtained from different enzyme types and levels. Carbohydr. Polym. 2017, 157, 533–540. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, W.; Li, X.; Zhang, J.; Huang, L. Effect of acid-ethanol on the physicochemical properties of Dioscorea opposita Thunb. and Pueraria thomsonii Benth. starches. Starch-Stärke 2011, 63, 302–310. [Google Scholar] [CrossRef]
- Chen, B.; Dang, L.; Zhang, X.; Fang, W.; Hou, M.; Liu, T.; Wang, Z. Physicochemical properties and micro-structural characteristics in starch from kudzu root as affected by cross-linking. Food Chem. 2017, 219, 93–101. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Lu, F.; Zhang, L.; Yang, Q.; Mu, J.; Li, X. Physicochemical properties of corn starch isolated by acid liquid and l-cysteine. Food Hydrocoll. 2015, 44, 353–359. [Google Scholar] [CrossRef]
- Shang, X.; Wang, Q.; Li, J.; Zhang, G.; Zhang, J.; Liu, P.; Wang, L. Double-network hydrogels with superior self-healing properties using starch reinforcing strategy. Carbohydr. Polym. 2021, 257, 117626. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch-Stärke 2015, 67, 55–68. [Google Scholar] [CrossRef]
- Chang, R.; Ji, N.; Li, M.; Qiu, L.; Sun, C.; Bian, X.; Qiu, H.; Xiong, L.; Sun, Q. Green preparation and characterization of starch nanoparticles using a vacuum cold plasma process combined with ultrasonication treatment. Ultrason. Sonochem. 2019, 58, 104660. [Google Scholar] [CrossRef]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CYTA J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Song, X.; Dong, H.; Zang, Z.; Wu, W.; Zhu, W.; Zhang, H.; Guan, Y. Kudzu resistant starch: An effective regulator of type 2 diabetes mellitus. Oxid. Med. Cell. Longev. 2021, 2021, 4448048. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. In-vitro digestibility, rheology, structure, and functionality of RS3 from oat starch. Food Chem. 2016, 212, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Shin, M. Improving gel formation of rice starch added with cross-linked resistant starch prepared from rice starch. Starch-Stärke 2015, 67, 829–837. [Google Scholar] [CrossRef]
- Campbell, J. High-Throughput Assessment of Bacterial Growth Inhibition by Optical Density Measurements. Curr. Protoc. Chem. Biol. 2010, 2, 195–208. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Appiah, A.P.; Torku, G.D.; Darko, A.Y.; Wiredu, A. Determination of methanol and ethanol concentrations in local and foreign alcoholic drinks and food products (Banku, Ga kenkey, Fante kenkey and Hausa koko) in Ghana. Int. J. Food Contam. 2017, 4, 1–5. [Google Scholar] [CrossRef]




| Simulated Fluids | SSF (Mouth) | SGF (Stomach) | SIF (Intestine) | |||
|---|---|---|---|---|---|---|
| Constituent | (mM) | mL | (mM) | mL | (mM) | mL |
| KCl | 15.1 | 15.1 | 6.9 | 6.9 | 6.8 | 6.8 |
| KH2PO4 | 3.7 | 3.7 | 0.9 | 0.9 | 0.8 | 0.8 |
| NaHCO3 | 13.6 | 6.8 | 25 | 12.5 | 85 | 42.5 |
| NaCl | - | - | 47.2 | 11.8 | 38.4 | 9.6 |
| MgCl2(H2O)6 | 0.15 | 0.5 | 0.12 | 0.4 | 0.33 | 1.1 |
| (NH4)2CO3 | 0.06 | 0.06 | 0.5 | 0.5 | - | - |
| HCl | 1.1 | 0.09 | 15.6 | 1.3 | 8.4 | 0.7 |
| CaCl2(H2O)2 | 1.5 | 0.025 | 0.15 | 0.005 | 0.6 | 0.04 |
| Samples | Textural Characteristics | Fitted Rheological Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| EC | ET | Hardness (g) | Springiness | Resilience | Chewiness (g) | k | n | R2 |
| 0% | 62.22 ± 1.52 a | 0.54 ± 0.02 a | 61.31 ± 1.61 a | 52.81 ± 1.98 a | 1055 ± 73.01 g | −0.63 ± 0.02 b | 0.974 d | |
| 30% | 2 h | 154.55 ± 2.01 b | 0.61 ± 0.02 b | 73.58 ± 3.15 b | 57.08 ± 0.99 b | 2235 ± 150 c | −0.73 ± 0.03 ef | 0.963 g |
| 60% | 153.52 ± 1.52 b | 0.73 ± 0.04 c | 73.95 ± 1.37 b | 70.52 ± 1.22 ed | 2145 ± 105 cd | −0.71 ± 0.02 e | 0.978 bc | |
| 80% | 165.08 ± 2.23 c | 0.73 ± 0.01 c | 75.41 ± 2.11 c | 71.18 ± 3.01 e | 1999 ± 137 e | −0.69 ± 0.02 d | 0.973 d | |
| 100% | 168.62 ± 3.11 cd | 0.79 ± 0.03 d | 74.82 ± 1.37 bc | 73.02 ± 1.86 ef | 2867 ± 124 b | −0.89 ± 0.02 g | 0.990 a | |
| 30% | 4 h | 157.25 ± 1.01 b | 0.63 ± 0.02 b | 74.58 ± 2.05 bc | 58.21 ± 0.98 b | 661 ± 30 h | −0.58 ± 0.01 a | 0.977 c |
| 60% | 164.05 ± 1.61 c | 0.79 ± 0.04 d | 78.23 ± 1.66 d | 69.98 ± 1.81 d | 1932 ± 205 e | −0.66 ± 0.04 c | 0.965 f | |
| 80% | 169.29 ± 2.41 d | 0.81 ± 0.01 de | 81.16 ± 1.31 f | 75.22 ± 3.44 f | 3698 ± 55 a | −1.62 ± 0.07 h | 0.955 h | |
| 100% | 177.37 ± 1.22 f | 0.98 ± 0.03 b | 79.15 ± 2.18 e | 79.11 ± 1.61 g | 1358 ± 65 f | −0.67 ± 0.01 cd | 0.979 bc | |
| 30% | 6 h | 155.08 ± 1.05 b | 0.71 ± 0.02 c | 80.82 ± 3.25 ef | 63.71 ± 1.00 c | 2149 ± 104 c | −0.70 ± 0.02 de | 0.980 b |
| 60% | 169.53 ± 1.84 d | 0.83 ± 0.04 e | 85.23 ± 1.63 g | 69.45 ± 1.35 d | 1991 ± 136 e | −0.69 ± 0.02 d | 0.982 ab | |
| 80% | 171.51 ± 2.23 e | 0.97 ± 0.01 f | 89.91 ± 2.07 h | 79.89 ± 2.03 g | 2102 ± 121 d | −0.62 ± 0.02 b | 0.971 e | |
| 100% | 178.82 ± 1.69 f | 1.01 ± 0.03 g | 91.15 ± 2.23 h | 79.89 ± 1.55 c | 1045 ± 74 g | −0.65 ± 0.02 c | 0.966 f | |
| Samples | Thermal Characteristics | Digestibility | Others | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC | ET | To (°C) | Tp (°C) | Tc (°C) | Tc-To (°C) | ΔHg (J/g) | DS % | RS % | S % | RC % |
| (0%) | 45.32 ± 0.1 b | 53.92 ± 0.3 b | 66.92 ± 0.3 b | 8.83 ± 0.1 a | 1.02 ± 0.3 f | 98.15 ± 0.43 a | 1.87 ± 0.12 h | 11.00 ± 0.1 a | 4.73 ± 0.3 j | |
| 30% | 2 h | 45.32 ± 0.2 b | 58.92 ± 0.3 b | 64.92 ± 0.3 b | 7.83 ± 0.1 a | 2.92 ± 0.3 b | 95.08 ± 0.65 b | 4.92 ± 0.19 g | 11.03 ± 0.0 a | 5.21 ± 0.1 i |
| 60% | 45.80 ± 0.4 b | 60.95 ± 0.1 b | 65.95 ± 0.1 b | 7.01 ± 0.6 c | 2.81 ± 0.1 ab | 93.75 ± 0.83 c | 6.25 ± 0.23 d | 10.04 ± 0.3 b | 5.93 ± 0.4 g | |
| 80% | 46.41 ± 0.5 a | 61.89 ± 0.1 b | 65.89 ± 0.1 b | 7.11 ± 0.3 b | 2.79 ± 0.1 b | 92.67 ± 0.36 c | 7.92 ± 0.52 bc | 9.60 ± 0.1 c | 6.82 ± 0.2 e | |
| 100% | 42.09 ± 0.7 d | 50.97 ± 0.3 b | 63.97 ± 0.3 b | 7.28 ± 0.2 c | 2.97 ± 0.3 a | 92.19 ± 0.88 cd | 7.81 ± 0.18 bc | 7.12 ± 0.2 g | 7.25 ± 0.3 c | |
| 30% | 4 h | 43.12 ± 0.4 c | 59.53 ± 0.2 b | 65.53 ± 0.2 b | 6.54 ± 0.5 b | 2.53 ± 0.2 c | 94.92 ± 0.91 b | 5.09 ± 0.21 e | 8.41 ± 0.1 d | 5.74.7 ± 0.1 h |
| 60% | 45.32 ± 0.1 b | 52.92 ± 0.3 b | 62.92 ± 0.3 b | 6.83 ± 0.1 a | 2.12 ± 0.3 b | 93.61 ± 0.57 c | 6.39 ± 0.47 d | 7.92 ± 0.1 e | 6.23 ± 0.2 f | |
| 80% | 43.10 ± 0.3 c | 62.95 ± 0.1 b | 69.95 ± 0.1 b | 6.01 ± 0.6 c | 2.25 ± 0.1 cd | 92.31 ± 0.72 d | 7.69 ± 0.29 c | 7.43 ± 0.4 f | 7.11 ± 0.0 d | |
| 100% | 41.41 ± 0.5 e | 59.89 ± 0.1 b | 63.89 ± 0.1 b | 5.11 ± 0.3 b | 2.09 ± 0.1 f | 91.93 ± 0.73 e | 8.05 ± 0.34 b | 6.55 ± 0.0 i | 7.59 ± 0.1 b | |
| 30% | 6 h | 42.09 ± 0.5 d | 58.97 ± 0.3 b | 63.97 ± 0.3 b | 6.28 ± 0.2 c | 1.98 ± 0.3 b | 94.96 ± 0.49 b | 5.03 ± 0.35 f | 7.31 ± 0.2 fg | 5.92 ± 0.0 f |
| 60% | 41.12 ± 0.2 e | 61.53 ± 0.2 b | 64.53 ± 0.2 b | 5.82 ± 0.5 b | 2.35 ± 0.2 d | 92.91 ± 0.99 c | 7.07 ± 0.52 c | 7.19 ± 0.1 g | 6.74 ± 0.0 e | |
| 80% | 43.09 ± 0.3 c | 63.97 ± 0.3 b | 11.97 ± 0.3 b | 6.28 ± 0.2 c | 2.17 ± 0.3 e | 91.87 ± 0.94 d | 8.14 ± 0.33 a | 6.72 ± 0.3 h | 7.51 ± 0.2 b | |
| 100% | 41.12 ± 0.4 e | 53.53 ± 0.2 b | 16.53 ± 0.2 b | 6.52 ± 0.5 b | 2.83 ± 0.2 b | 91.27 ± 0.81 d | 8.73 ± 0.25 a | 5.96 ± 0.3 j | 7.90 ± 0.1 a | |
| Syneresis | RC | Hardness | Springiness | Resilience | Chewiness | Size | DS | RS | L* | a* | b* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Syneresis | 1 | |||||||||||
| RC | 0.725 | 1 | ||||||||||
| Hardness | 0.689 | 0.537 | 1 | |||||||||
| Springiness | 0.692 * | 0.672 | 0.820 * | 1 | ||||||||
| Resilience | 0.753 * | −0.599 | 0.863 * | 0.795 * | 1 | |||||||
| Chewiness | 0.521 | 0.659 | 0.699 | 0.574 | 0.894 * | 1 | ||||||
| Size | 0.746 | −0.371 | −0.592 | 0.498 | −0.569 | 0.632 | 1 | |||||
| DS | −0.649 | −0.823 * | −0.516 | 0.564 | 0.628 | −0.748 | 0.297 | 1 | ||||
| RS | 0.853 * | 0.795 * | 0.647 | 0.594 | 0.774 * | 0.539 | 0.211 | −0.845 * | 1 | |||
| L* | 0.572 | 0.439 | 0.154 | 0.473 * | −0.367 | 0.255 | 0.527 | 0.319 | 0.198 | 1 | ||
| a* | −0.434 | 0.255 | 0.316 | −0.259 | 0.401 | 0.374 * | 0.498 | 0.225 | 0.254 | −0.789 * | 1 | |
| b* | −0.413 | 0.398 * | 0.299 | 0.325 | 0.384 | 0.296 | 0.596 | 0.497 | 0.352 | 0.522 | 0.658 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekumah, J.-N.; Han, X.; Liang, Q.; Kang, L.; Wei, B.; Rashid, A.; Virk, M.S.; Qayum, A.; Adade, S.Y.-S.S.; Johnson, N.A.N.; et al. Production of Kudzu Starch Gels with Superior Mechanical and Rheological Properties through Submerged Ethanol Exposure and Implications for In Vitro Digestion. Foods 2023, 12, 3992. https://doi.org/10.3390/foods12213992
Ekumah J-N, Han X, Liang Q, Kang L, Wei B, Rashid A, Virk MS, Qayum A, Adade SY-SS, Johnson NAN, et al. Production of Kudzu Starch Gels with Superior Mechanical and Rheological Properties through Submerged Ethanol Exposure and Implications for In Vitro Digestion. Foods. 2023; 12(21):3992. https://doi.org/10.3390/foods12213992
Chicago/Turabian StyleEkumah, John-Nelson, Xu Han, Qiufang Liang, Lixin Kang, Benxi Wei, Arif Rashid, Muhammad Safiullah Virk, Abdul Qayum, Selorm Yao-Say Solomon Adade, Nana Adwoa Nkuma Johnson, and et al. 2023. "Production of Kudzu Starch Gels with Superior Mechanical and Rheological Properties through Submerged Ethanol Exposure and Implications for In Vitro Digestion" Foods 12, no. 21: 3992. https://doi.org/10.3390/foods12213992
APA StyleEkumah, J. -N., Han, X., Liang, Q., Kang, L., Wei, B., Rashid, A., Virk, M. S., Qayum, A., Adade, S. Y. -S. S., Johnson, N. A. N., & Ren, X. (2023). Production of Kudzu Starch Gels with Superior Mechanical and Rheological Properties through Submerged Ethanol Exposure and Implications for In Vitro Digestion. Foods, 12(21), 3992. https://doi.org/10.3390/foods12213992