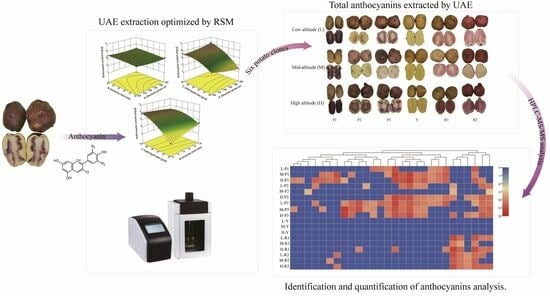
Anthocyanin Profiles in Colored Potato Tubers at Different Altitudes by HPLC–MS Analysis with Optimized Ultrasound-Assisted Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Preparations
2.3. Data on Climatic Factors
2.4. Ultrasound-Assisted Extraction
2.5. Total Anthocyanin Quantification
2.6. Single-Factor Tests for Total Anthocyanin Extraction
2.7. Optimization of Anthocyanin Extraction
2.8. Purification of Anthocyanins from Potato Tubers
2.9. Identification of Anthocyanins Using HPLC–MS
2.10. Statistical Analysis
3. Results
3.1. Single-Factor Analysis of Anthocyanin Extraction
3.2. Regression Equation Establishment and Analysis of Variance
3.3. Model Optimization and Verification
3.4. Anthocyanin Yield Obtained Using the Optimized Ultrasonic Method
3.5. Analysis of Anthocyanin Composition of Potatoes at Different Altitudes Using HPLC–MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Zhang, N.; Fu, X.; Zhang, H.; Liu, S.; Pu, X.; Wang, X.; Si, H. StTCP15 Regulates Potato Tuber Sprouting by Modulating the Dynamic Balance between Abscisic Acid and Gibberellic Acid. Front. Plant Sci. 2022, 13, 1009552. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and Human Health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and Anthocyanin-Rich Extracts: Role in Diabetes and Eye Function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- Tsuda, T. Regulation of Adipocyte Function by Anthocyanins; Possibility of Preventing the Metabolic Syndrome. J. Agric. Food Chem. 2008, 56, 642–646. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional Regulation of Anthocyanin Biosynthesis in Ripening Fruits of Grapevine under Seasonal Water Deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.; Xu, R.; Wang, J.; Lin, Y.; Pang, J.; Wu, S.; Zhong, F. Comprehensive Analysis of Photosynthetic Characteristics and Quality Improvement of Purple Cabbage under Different Combinations of Monochromatic Light. Front. Plant Sci. 2016, 7, 1788. [Google Scholar] [CrossRef]
- Camel, V. Microwave-Assisted Solvent Extraction of Environmental Samples. Trends Anal. Chem. 2000, 19, 229–248. [Google Scholar] [CrossRef]
- Ni, J.; Ding, C.; Zhang, Y.; Song, Z.; Hu, X.; Hao, T. Electrohydrodynamic Drying of Chinese Wolfberry in a Multiple Needle-to-Plate Electrode System. Foods 2019, 8, 152. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of Extraction of Natural Products Using Ultrasonic Irradiations—A Review of Current Status. Chem. Eng. Process. Process Intensif. 2010, 49, 10–23. [Google Scholar] [CrossRef]
- Leksawasdi, N.; Taesuwan, S.; Prommajak, T.; Techapun, C.; Khonchaisri, R.; Sittilop, N.; Halee, A.; Jantanasakulwong, K.; Phongthai, S.; Nunta, R.; et al. Ultrasonic Extraction of Bioactive Compounds from Green Soybean Pods and Application in Green Soybean Milk Antioxidants Fortification. Foods 2022, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xue, H.; Li, J. Extraction of Phenolics and Anthocyanins from Purple Eggplant Peels by Multi-Frequency Ultrasound: Effects of Different Extraction Factors and Optimization Using Uniform Design. Ultrason. Sonochem. 2022, 90, 106174. [Google Scholar] [CrossRef] [PubMed]
- Pinho, C.; Melo, A.; Mansilha, C.; Ferreira, I.M.P.L.V.O. Optimization of Conditions for Anthocyanin Hydrolysis from Red Wine Using Response Surface Methodology (RSM). J. Agric. Food Chem. 2011, 59, 50–55. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Ramli, M.E.; Salleh, R.M. Optimum Extraction Condition of Clitorea Ternatea Flower on Antioxidant Activities, Total Phenolic, Total Flavonoid and Total Anthocyanin Contents. Trop. Life Sci. Res. 2020, 31, 1. [Google Scholar] [CrossRef]
- Paulsmeyer, M.N.; Vermillion, K.E.; Juvik, J.A. Assessing the Diversity of Anthocyanin Composition in Various Tissues of Purple Corn (Zea mays L.). Phytochemistry 2022, 201, 113263. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, S.; Xu, Q.; Yan, C.; Yang, J.; Zhou, Y. Optimization and Application of HPLC for Simultaneous Separation of Six Well-Known Major Anthocyanins in Blueberry. Prep. Biochem. Biotechnol. 2021, 51, 961–970. [Google Scholar] [CrossRef]
- Buendía, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.M.; Tomás-Barberán, F.A. HPLC-MS Analysis of Proanthocyanidin Oligomers and Other Phenolics in 15 Strawberry Cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS Quantification of Anthocyanins and Non-Anthocyanin Phenolics from Blueberries with Widely Divergent Profiles and Biological Activies. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef]
- Li, L.; Zuo, Z.; Wang, Y. Practical Qualitative Evaluation and Screening of Potential Biomarkers for Different Parts of Wolfiporia Cocos Using Machine Learning and Network Pharmacology. Front. Microbiol. 2022, 13, 931967. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and PH Degradation Kinetics of Anthocyanins in Natural Food Colorant Prepared from Black Rice Bran. J. Food Sci. Technol. 2016, 53, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Liu, Z.; Wang, L.; Bi, Z.; Sun, C.; Yao, P.; Zhang, J.; Bai, J.; Zeng, Y. Integrated Transcriptomic and Metabolomic Analysis Revealed Altitude-Related Regulatory Mechanisms on Flavonoid Accumulation in Potato Tubers. Food Res. Int. 2023, 170, 112997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-Assisted Enzymatic Extraction and Identification of Anthocyanin Components from Mulberry Wine Residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef] [PubMed]
- Anthocyanin Extraction from Jaboticaba Skin (Myrciaria Cauliflora Berg.) Using Conventional and Non-Conventional Methods—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8954074/ (accessed on 10 November 2023).
- Ticconi, C.A.; Delatorre, C.A.; Abel, S. Attenuation of Phosphate Starvation Responses by Phosphite in Arabidopsis. Plant Physiol. 2001, 127, 963–972. [Google Scholar] [CrossRef]
- Zou, T.-B.; Wang, M.; Gan, R.-Y.; Ling, W.-H. Optimization of Ultrasound-Assisted Extraction of Anthocyanins from Mulberry, Using Response Surface Methodology. Int. J. Mol. Sci. 2011, 12, 3006–3017. [Google Scholar] [CrossRef]
- Cao, X.; Qiu, Z.; Wang, X.; Van Giang, T.; Liu, X.; Wang, J.; Wang, X.; Gao, J.; Guo, Y.; Du, Y.; et al. A Putative R3 MYB Repressor Is the Candidate Gene Underlying Atroviolacium, a Locus for Anthocyanin Pigmentation in Tomato Fruit. J. Exp. Bot. 2017, 68, 5745–5758. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Q.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Cravotto, G.; Yang, X.; Li, S.; He, J. HPLC-DAD-ESI-MS2 Analytical Profile of Extracts Obtained from Purple Sweet Potato after Green Ultrasound-Assisted Extraction. Food Chem. 2017, 215, 391–400. [Google Scholar] [CrossRef]
- Liu, Y.; Zhe, W.; Zhang, R.; Peng, Z.; Wang, Y.; Gao, H.; Guo, Z.; Xiao, J. Ultrasonic-Assisted Extraction of Polyphenolic Compounds from Paederia scandens (Lour.) Merr. Using Deep Eutectic Solvent: Optimization, Identification, and Comparison with Traditional Methods. Ultrason. Sonochem. 2022, 86, 106005. [Google Scholar] [CrossRef]
- Avhad, D.N.; Rathod, V.K. Ultrasound Assisted Production of a Fibrinolytic Enzyme in a Bioreactor. Ultrason. Sonochem. 2015, 22, 257–264. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Angolini, C.F.F.; Eberlin, M.N.; Meireles, M.A.A.; Pastore, G.M. Effects of High-Intensity Ultrasound Process Parameters on the Phenolic Compounds Recovery from Araticum Peel. Ultrason. Sonochem. 2019, 50, 82–95. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of Low Power Ultrasound-Assisted Extraction of Phenolic Compounds from Mandarin (Citrus reticulata Blanco Cv. Sainampueng) Peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Velasco González de Peredo, A.; Vázquez Espinosa, M.; Espada Bellido, E.; FerreiroGonzález, M.; Carrera Fernández, C.; Palma Lovillo, M.; Alvarez Saura, J.A.; Fernández Barbero, G.; Ayuso Vilacides, J. Optimization of Analytical Ultrasound-Assisted Methods for the Extraction of Total Phenolic Compounds and Anthocyanins from Sloes (Prunus spinosa L.). Agronomy 2020, 10, 966. [Google Scholar] [CrossRef]
- Blackhall, M.L.; Berry, R.; Davies, N.W.; Walls, J.T. Optimized Extraction of Anthocyanins from Reid Fruits’ Prunus Avium “Lapins” Cherries. Food Chem. 2018, 256, 280–285. [Google Scholar] [CrossRef]
- Shafi, J.; Sun, Z.; Ji, M.; Gu, Z.; Ahmad, W. ANN and RSM Based Modelling for Optimization of Cell Dry Mass of Bacillus Sp. Strain B67 and Its Antifungal Activity against Botrytis cinerea. Biotechnol. Biotechnol. Equip. 2017, 32, 58–68. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.-X.; Zhang, Z.; Fang, Y.; Li, D.; Chen, X.-S.; Feng, S.-Q. Ethylene Precisely Regulates Anthocyanin Synthesis in Apple via a Module Comprising MdEIL1, MdMYB1, and MdMYB17. Hortic. Res. 2022, 9, uhac034. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhang, D.-L.; Sun, B.-Y.; Liu, S.-M. Evaluating the Impacts of Climate Factors and Flavonoids Content on Chinese Prickly Ash Peel Color Based on HPLC-MS and Structural Equation Model. Foods 2022, 11, 2539. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Tanou, G.; Scossa, F.; Sarrou, E.; Stamatakis, G.; Samiotaki, M.; Martens, S.; Fernie, A.R.; Molassiotis, A. Decoding Altitude-Activated Regulatory Mechanisms Occurring during Apple Peel Ripening. Hortic. Res. 2020, 7, 120. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Apichai, S.; Sirilun, S.; Julsrigival, J.; Sawangrat, K.; Ogata, F.; Kawasaki, N.; Sirithunyalug, B.; Saenjum, C. Anthocyanin Profile, Antioxidant, Anti-Inflammatory, and Antimicrobial against Foodborne Pathogens Activities of Purple Rice Cultivars in Northern Thailand. Molecules 2021, 26, 5234. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, G.; Tian, L.; Wu, H.; Ren, Y.; Tamir, G.; Huang, W.; Yu, H. High Altitude Is Beneficial for Antioxidant Components and Sweetness Accumulation of Rabbiteye Blueberry. Front. Plant Sci. 2020, 11, 573531. [Google Scholar] [CrossRef]
- Carrera, C.; Aliaño-González, M.J.; Valaityte, M.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. A Novel Ultrasound-Assisted Extraction Method for the Analysis of Anthocyanins in Potatoes (Solanum tuberosum L.). Antioxidants 2021, 10, 1375. [Google Scholar] [CrossRef]
- Ceci, A.T.; Franceschi, P.; Serni, E.; Perenzoni, D.; Oberhuber, M.; Robatscher, P.; Mattivi, F. Metabolomic Characterization of Pigmented and Non-Pigmented Potato Cultivars Using a Joint and Individual Variation Explained (JIVE). Foods 2022, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Epl, J. Impact of Selected Factors—Cultivar, Storage, Cooking and Baking on the Content of Anthocyanins in Coloured-Flesh Potatoes. Food Chem. 2012, 133, 1107–1116. [Google Scholar] [CrossRef]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Phenolic Compounds and Antioxidant Activities of Potato Cultivars with White, Yellow, Red and Purple Flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef] [PubMed]




| Factor Levels | Independent Variable | ||
|---|---|---|---|
| Ultrasonic Power (A) | Extraction Time (B) | Solid-Liquid Ratio(C) | |
| 1 | 300 | 20 | 1:30 |
| 0 | 250 | 15 | 1:25 |
| −1 | 200 | 10 | 1:20 |
| Run | Extraction Condition | Anthocyanin Content (mg/g) | |||
|---|---|---|---|---|---|
| A | B | C | Actual Value | Predicted Value | |
| 1 | 1 (300) | −1 (10) | 0 (1:25) | 3.11 | 3.02 |
| 2 | 1 (300) | 1 (20) | 0 (1:25) | 3.4 | 3.44 |
| 3 | 1 (300) | 0 (15) | 1 (1:30) | 4.24 | 4.15 |
| 4 | 0 (250) | 1 (20) | −1 (1:20) | 2.57 | 2.39 |
| 5 | −1 (200) | 1 (20) | 0 (1:25) | 3.4 | 3.49 |
| 6 | 0 (250) | 0 (15) | 0 (1:25) | 3.11 | 3.26 |
| 7 | −1 (200) | 0 (15) | −1 (1:20) | 2.41 | 2.49 |
| 8 | 0 (250) | −1 (10) | −1 (1:20) | 1.34 | 1.29 |
| 9 | 0 (250) | −1 (10) | 1 (1:30) | 3.77 | 3.94 |
| 10 | 1 (300) | 0 (15) | −1 (1:20) | 1.37 | 1.51 |
| 11 | −1 (200) | −1 (10) | 0 (1:25) | 3.36 | 3.33 |
| 12 | 0 (250) | 1 (20) | 1 (1:30) | 3.37 | 3.42 |
| 13 | 0 (250) | 0 (15) | 0 (1:25) | 3.38 | 3.26 |
| 14 | 0 (250) | 0 (15) | 0 (1:25) | 3.39 | 3.26 |
| 15 | 0 (250) | 0 (15) | 0 (1:25) | 3.13 | 3.26 |
| 16 | 0 (250) | 0 (15) | 0 (1:25) | 3.27 | 3.26 |
| 17 | −1 (200) | 0 (15) | 1 (1:30) | 3.68 | 3.54 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 9.22 | 9 | 1.02 | 33.83 | <0.0001 | significant |
| A | 0.0666 | 1 | 0.0666 | 2.20 | 0.1817 | |
| B | 0.1682 | 1 | 0.1682 | 5.55 | 0.0506 | |
| C | 6.79 | 1 | 6.79 | 224.08 | <0.0001 | significant |
| AB | 0.0156 | 1 | 0.0156 | 0.5157 | 0.4959 | |
| AC | 0.6400 | 1 | 0.6400 | 21.12 | 0.0025 | |
| BC | 0.6642 | 1 | 0.6642 | 21.92 | 0.0023 | |
| A2 | 0.0528 | 1 | 0.0528 | 1.74 | 0.2283 | |
| B2 | 0.0107 | 1 | 0.0107 | 0.3544 | 0.5704 | |
| C2 | 0.8263 | 1 | 0.8263 | 27.27 | 0.0012 | |
| Residual | 0.2121 | 7 | 0.0303 | |||
| Lack of Fit | 0.1414 | 3 | 0.0471 | 2.67 | 0.1836 | not significant |
| Pure Error | 0.0707 | 4 | 0.0177 | |||
| R2 | 0.9775 | |||||
| R2 (Adj) | 0.9486 | |||||
| C.V.% | 5.66 | |||||
| Cor Total | 9.44 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Wang, W.; Liu, Z.; Niu, N.; Li, Z.; Chen, L.; Zhu, J.; Li, D.; Liu, Y. Anthocyanin Profiles in Colored Potato Tubers at Different Altitudes by HPLC–MS Analysis with Optimized Ultrasound-Assisted Extraction. Foods 2023, 12, 4175. https://doi.org/10.3390/foods12224175
Qi Z, Wang W, Liu Z, Niu N, Li Z, Chen L, Zhu J, Li D, Liu Y. Anthocyanin Profiles in Colored Potato Tubers at Different Altitudes by HPLC–MS Analysis with Optimized Ultrasound-Assisted Extraction. Foods. 2023; 12(22):4175. https://doi.org/10.3390/foods12224175
Chicago/Turabian StyleQi, Zheying, Weilu Wang, Zhen Liu, Na Niu, Zhitao Li, Limin Chen, Jinyong Zhu, Dechen Li, and Yuhui Liu. 2023. "Anthocyanin Profiles in Colored Potato Tubers at Different Altitudes by HPLC–MS Analysis with Optimized Ultrasound-Assisted Extraction" Foods 12, no. 22: 4175. https://doi.org/10.3390/foods12224175
APA StyleQi, Z., Wang, W., Liu, Z., Niu, N., Li, Z., Chen, L., Zhu, J., Li, D., & Liu, Y. (2023). Anthocyanin Profiles in Colored Potato Tubers at Different Altitudes by HPLC–MS Analysis with Optimized Ultrasound-Assisted Extraction. Foods, 12(22), 4175. https://doi.org/10.3390/foods12224175