A Novel Strategy to Improve Cloud Stability of Orange-Based Juice: Combination of Natural Pectin Methylesterase Inhibitor and High-Pressure Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Physiochemical Properties
2.4. Rheological Properties
2.5. Particle Size Distribution
2.6. Enzyme Activities
2.6.1. PME Activity
2.6.2. PMEI Inhibitory Activity
2.7. Pectin Characterization
2.7.1. Alcohol-Insoluble Residue Extraction and Fractionation
2.7.2. Degree of Esterification Analysis
2.7.3. Molar Mass Distribution Measurement
2.7.4. GalA Content Measurement
2.8. Data Analysis
3. Results and Discussion
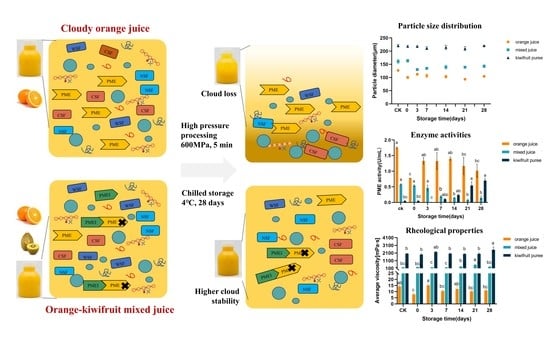
3.1. Visual Appearance
3.2. Physicochemical Properties
3.2.1. pH, TSS, and TA Values
3.2.2. Particle Size Distribution
3.2.3. Rheological Properties
3.3. Enzyme Activity
3.4. Pectin Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agcam, E.; Akyildiz, A.; Akdemir Evrendilek, G. A Comparative Assessment of Long-Term Storage Stability and Quality Attributes of Orange Juice in Response to Pulsed Electric Fields and Heat Treatments. Food Bioprod. Process. 2016, 99, 90–98. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Q.; Yuan, Q.; Gao, C.; Ge, Q.; Li, C.; Liu, X.; Ma, T. Thermosonication Combined with ε-Polylysine (TSε): A Novel Technology to Control the Microbial Population and Significantly Improve the Overall Quality Attributes of Orange Juice. Food Control 2022, 141, 109200. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Zhang, M.; Yin, Y.; Liu, Z.; Hu, X.; Yi, J. Effect of Centrifugal Pre-Treatment on Flavor Change of Cloudy Orange Juice: Interaction between Pectin and Aroma Release. Food Chem. 2022, 374, 131705. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.T.; Hai Dang, D.N.; Buvé, C.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M. Quality Change during High Pressure Processing and Thermal Processing of Cloudy Apple Juice. LWT 2017, 75, 85–92. [Google Scholar] [CrossRef]
- Atuonwu, J.C.; Tassou, S.A. Model-Based Energy Performance Analysis of High Pressure Processing Systems. Innov. Food Sci. Emerg. Technol. 2018, 47, 214–224. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Ziaiifar, A.M. A Review of Pectin Methylesterase Inactivation in Citrus Juice during Pasteurization. Trends Food Sci. Technol. 2018, 71, 1–12. [Google Scholar] [CrossRef]
- Croak, S.; Corredig, M. The Role of Pectin in Orange Juice Stabilization: Effect of Pectin Methylesterase and Pectinase Activity on the Size of Cloud Particles. Food Hydrocoll. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Zhao, L.; Rao, L.; Wang, Y.; Liao, X. Inhibition Effect of High Hydrostatic Pressure Combined with Epigallocatechin Gallate Treatments on Pectin Methylesterase in Orange Juice and Model System. Food Chem. 2022, 390, 133147. [Google Scholar] [CrossRef]
- Jolie, R.P.; Duvetter, T.; Van Loey, A.M.; Hendrickx, M.E. Pectin Methylesterase and Its Proteinaceous Inhibitor: A Review. Carbohydr. Res. 2010, 345, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Matteo, D.A. Structural Basis for the Interaction between Pectin Methylesterase and a Specific Inhibitor Protein. Plant Cell 2005, 17, 849–858. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Bi, J.; McClements, D.J.; Liu, X.; Yi, J.; Lyu, J.; Zhou, M.; Verkerk, R.; Dekker, M.; Wu, X.; et al. Impacts of Thermal and Non-Thermal Processing on Structure and Functionality of Pectin in Fruit-and Vegetable-Based Products: A Review. Carbohydr. Polym. 2020, 250, 116890. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, W.; Wang, Y.; Xu, Y.; Zhang, W.; Lao, F.; Liao, X.; Wu, J. Physicochemical and Structural Properties of Three Pectin Fractions from Muskmelon (Cucumis Melo) and Their Correlation with Juice Cloud Stability. Food Hydrocoll. 2022, 124, 107313. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a Rheology Modifier: Origin, Structure, Commercial Production and Rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.T.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M. A Multivariate Approach into Physicochemical, Biochemical and Aromatic Quality Changes of Purée Based on Hayward Kiwifruit during the Final Phase of Ripening. Postharvest Biol. Technol. 2016, 117, 206–216. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Guo, C.; Hu, X.; Yi, J. Role of Pectin Characteristics in Orange Juice Stabilization: Effect of High-Pressure Processing in Combination with Centrifugation Pretreatments. Int. J. Biol. Macromol. 2022, 215, 615–624. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, X.; Zheng, Y.; Wang, D.; Deng, Y.; Zhao, Y. Impact of Ultrasonication/Shear Emulsifying/Microwave-Assisted Enzymatic Extraction on Rheological, Structural, and Functional Properties of Akebia Trifoliata (Thunb.) Koidz. Seed Protein Isolates. Food Hydrocoll. 2021, 112, 106355. [Google Scholar] [CrossRef]
- Jolie, R.P.; Duvetter, T.; Houben, K.; Clynen, E.; Sila, D.N.; Van Loey, A.M.; Hendrickx, M.E. Carrot Pectin Methylesterase and Its Inhibitor from Kiwi Fruit: Study of Activity, Stability and Inhibition. Innov. Food Sci. Emerg. Technol. 2009, 10, 601–609. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Feng, W.; Zhang, Z.; Li, H. Structural Characterization and Immunomodulatory Activities of Two Polysaccharides from Rehmanniae Radix Praeparata. Int. J. Biol. Macromol. 2021, 186, 385–395. [Google Scholar] [CrossRef]
- Genovese, D.B.; Lozano, J.E. Contribution of Colloidal Forces to the Viscosity and Stability of Cloudy Apple Juice. Food Hydrocoll. 2006, 20, 767–773. [Google Scholar] [CrossRef]
- Koh, J.; Morales-Contreras, B.E.; Guerra-Rosas, M.I.; Osorio-Hernández, E.; Culver, C.A.; Morales-Castro, J.; Wicker, L. Huanglongbing Disease and Quality of Pectin and Fruit Juice Extracted from Valencia Oranges. LWT 2020, 131, 109692. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.; Kristiani, K.; Buvé, C.; Van Loey, A.; Grauwet, T.; Hendrickx, M. The Potential of Kiwifruit Puree as a Clean Label Ingredient to Stabilize High Pressure Pasteurized Cloudy Apple Juice during Storage. Food Chem. 2018, 255, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Kebede, B.; Kristiani, K.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Minimizing Quality Changes of Cloudy Apple Juice: The Use of Kiwifruit Puree and High Pressure Homogenization. Food Chem. 2018, 249, 202–212. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Zhang, W.; Zhang, L.; Zhou, L.; Cai, S.; Hu, X.; Yi, J. Evaluation of Quality Changes of Differently Formulated Cloudy Mixed Juices during Refrigerated Storage after High Pressure Processing. Curr. Res. Food Sci. 2021, 4, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Kebede, B.T.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M. Comparing the Impact of High-Pressure Processing and Thermal Processing on Quality of “Hayward” and “Jintao” Kiwifruit Purée: Untargeted Headspace Fingerprinting and Targeted Approaches. Food Bioprocess Technol. 2016, 9, 2059–2069. [Google Scholar] [CrossRef]
- Roobab, U.; Shabbir, M.A.; Khan, A.W.; Arshad, R.N.; Bekhit, A.E.-D.; Zeng, X.-A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Treatments for Better Quality Clean-Label Juices and Beverages: Overview and Advances. LWT 2021, 149, 111828. [Google Scholar] [CrossRef]
- Fernández-Sestelo, A.; de Saá, R.S.; Pérez-Lamela, C.; Torrado-Agrasar, A.; Rúa, M.L.; Pastrana-Castro, L. Overall Quality Properties in Pressurized Kiwi Purée: Microbial, Physicochemical, Nutritive and Sensory Tests during Refrigerated Storage. Innov. Food Sci. Emerg. Technol. 2013, 20, 64–72. [Google Scholar] [CrossRef]
- Lv, R.; Kong, Q.; Mou, H.; Fu, X. Effect of Guar Gum on Stability and Physical Properties of Orange Juice. Int. J. Biol. Macromol. 2017, 98, 565–574. [Google Scholar] [CrossRef]
- Ricci, J.; Delalonde, M.; Wisniewski, C.; Dahdouh, L. Role of Dispersing and Dispersed Phases in the Viscoelastic Properties and the Flow Behavior of Fruit Juices during Concentration Operation: Case of Orange Juice. Food Bioprod. Process. 2021, 126, 121–129. [Google Scholar] [CrossRef]
- Patrignani, F.; Mannozzi, C.; Tappi, S.; Tylewicz, U.; Pasini, F.; Castellone, V.; Riciputi, Y.; Rocculi, P.; Romani, S.; Caboni, M.F.; et al. (Ultra) High Pressure Homogenization Potential on the Shelf-Life and Functionality of Kiwifruit Juice. Front. Microbiol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Kahraman, O.; Feng, H. Continuous-Flow Manothermosonication Treatment of Apple-Carrot Juice Blend: Effects on Juice Quality during Storage. LWT 2021, 137, 110360. [Google Scholar] [CrossRef]
- Demoulin, C.; Wisniewski, C.; Ricci, J.; Delalonde, M.; Dahdouh, L. Viscoelastic Behavior and Fouling Propensity of Concentrated Suspended Particles of Orange Juice with Defined Size Distributions: Towards a Better Control of the Deposit Layer Properties during Microfiltration. LWT 2022, 153, 112473. [Google Scholar] [CrossRef]
- Galant, A.L.; Widmer, W.W.; Luzio, G.A.; Cameron, R.G. Characterization of Molecular Structural Changes in Pectin during Juice Cloud Destabilization in Frozen Concentrated Orange Juice. Food Hydrocoll. 2014, 41, 10–18. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, Y.; Wei, L.; Xu, L.; Cao, X.; Liu, H.; Li, J. Effect of Particle Size on the Stability and Flavor of Cloudy Apple Juice. Food Chem. 2020, 328, 126967. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Vanga, S.K.; Raghavan, V. High-Intensity Ultrasound Processing of Kiwifruit Juice: Effects on the Microstructure, Pectin, Carbohydrates and Rheological Properties. Food Chem. 2020, 313, 126121. [Google Scholar] [CrossRef]
- Jolie, R.P.; Duvetter, T.; Houben, K.; Vandevenne, E.; Loey, A.; Declerck, P.J.; Hendrickx, M.E.; Gils, A. Plant Pectin Methylesterase and Its Inhibitor from Kiwi Fruit: Interaction Analysis by Surface Plasmon Resonance. Food Chem. 2010, 121, 207–214. [Google Scholar] [CrossRef]
- Welti-Chanes, J.; Ochoa-Velasco, C.E.; Guerrero-Beltrán, J.Á. High-Pressure Homogenization of Orange Juice to Inactivate Pectinmethylesterase. Innov. Food Sci. Emerg. Technol. 2009, 10, 457–462. [Google Scholar] [CrossRef]
- Duvetter, T.; Sila, D.N.; Buggenhout, S.V.; Jolie, R.; Hendrickx, M.E. Pectins in Processed Fruit and Vegetables: Part I—Stability and Catalytic Activity of Pectinases. Compr. Rev. Food Sci. Food Saf. 2010, 8, 75–85. [Google Scholar] [CrossRef]
- Yuliarti, O.; Matia-Merino, L.; Goh, K.T.; Mawson, J.; Brennan, C. Characterisation of Gold Kiwifruit Pectin Isolated by Enzymatic Treatment. Int. J. Food Sci. Technol. 2012, 47, 633–639. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative Study of the Cell Wall Composition of Broccoli, Carrot, and Tomato: Structural Characterization of the Extractable Pectins and Hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.L.R.; Rodrigues, P.D.; de Castro, R.J.S.; Silva, E.K. Sonoprocessing of Freshly Squeezed Orange Juice: Ascorbic Acid Content, Pectin Methylesterase Activity, Rheological Properties and Cloud Stability. Food Control. 2022, 131, 108391. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, F.; Yun, Y.; Tian, J.; Zhou, L. Gelation Behavior and Mechanism of Nicandra Physalodes (Linn.) Gaertn. Seeds Pectin Induced by Glucono–Delta–Lactone. Carbohydr. Polym. 2023, 299, 120151. [Google Scholar] [CrossRef]








| Storage Time (Days) | Color | pH | TSS (°Brix) | TA (%) | ||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE* | |||||
| Orange juice | Control | 58.28 ± 0.23 cde | 6.44 ± 0.30 a | 51.07 ± 0.52 a | – * | 4.35 ± 0.09 a | 8.70 ± 0.00 a | 0.31 ± 0.00 a |
| 0 | 57.97 ± 0.33 de | 3.93 ± 0.16 cd | 45.68 ± 0.25 b | 5.50 ± 0.27 a | 4.38 ± 0.03 a | 8.50 ± 0. 26 ab | 0.28 ± 0.00 c | |
| 3 | 59.15 ± 0.62 bc | 4.81 ± 1.25 bc | 45.81 ± 1.29 b | 1.23 ± 0.14 c | 4.27 ± 0.04 b | 8.63 ± 0.12 ab | 0.29 ± 0.01 bc | |
| 7 | 59.72 ± 0.40 ab | 4.73 ± 0.08 bc | 45.62 ± 0.96 b | 1.63 ± 0.22 bc | 4.32 ± 0.02 ab | 8.47 ± 0.05 b | 0.30 ± 0.01 ab | |
| 14 | 60.59 ± 0.96 a | 5.22 ± 0.37 b | 46.31 ± 1.19 b | 2.98 ± 0.82 a | 4.36 ± 0.01 a | 8.43 ± 0.05 b | 0.31 ± 0.00 a | |
| 21 | 57.80 ± 0.72 e | 3.67 ± 0.16 d | 43.64 ± 0.55 c | 2.08 ± 0.49 b | 4.35 ± 0.01 a | 8.43 ± 0.15 b | 0.30 ± 0.01 a | |
| 28 | 59.03 ± 0.67 bcd | 4.79 ± 0.04 bc | 46.23 ± 0.07 b | 1.36 ± 0.07 bc | 4.35 ± 0.01 a | 8.56 ± 0.05 ab | 0.30 ± 0.11 a | |
| Mixed juice | Control | 57.94 ± 0.11 a | 5.11 ± 0.08 cd | 50.07 ± 0.12 a | – * | 3.89 ± 0.05 a | 9.60 ± 0.00 b | 0.45 ± 0.00 bc |
| 0 | 56.93 ± 0.57 ab | 4.83 ± 0.34 d | 48.77 ± 0.66 ab | 1.67 ± 0.79 ab | 3.93 ± 0.08 a | 9.70 ± 0.00 a | 0.44 ± 0.01 c | |
| 3 | 56.19 ± 0.29 b | 4.92 ± 0.16 d | 47.53 ± 0.47 bc | 1.47 ± 0.51 ab | 3.90 ± 0.03 a | 9.63 ± 0.06 ab | 0.48 ± 0.01 a | |
| 7 | 57.04 ± 1.14 ab | 5.30 ± 0.32 cd | 46.98 ± 0.38 c | 2.13 ± 0.48 a | 3.89 ± 0.02 a | 9.68 ± 0.05 ab | 0.46 ± 0.01 bc | |
| 14 | 57.42 ± 0.75 a | 5.50 ± 0.49 bc | 47.66 ± 1.81 bc | 2.21 ± 0.63 a | 3.92 ± 0.02 a | 9.63 ± 0.06 ab | 0.45 ± 0.00 bc | |
| 21 | 57.74 ± 0.24 a | 6.08 ± 0.09 a | 49.23 ± 0.28 a | 1.49 ± 0.16 ab | 3.89 ± 0.03 a | 9.60 ± 0.00 b | 0.47 ± 0.01 ab | |
| 28 | 57.44 ± 0.26 a | 5.92 ± 0.15 ab | 49.17 ± 0.29 a | 1.29 ± 0.33 b | 3.95 ± 0.01 a | 9.48 ± 0.22 ab | 0.47 ± 0.00 ab | |
| Kiwifruit puree | Control | 61.46 ± 0.04 b | −1.46 ± 0.04 c | 27.29 ± 0.09 b | – * | 3.48 ± 0.02 b | 11.60 ± 0.00 ab | 0.84 ± 0.04 cd |
| 0 | 62.12 ± 0.06 a | −1.16 ± 0.02 a | 28.16 ± 0.07 a | 1.14 ± 0.11 e | 3.54 ± 0.02 a | 11.77 ± 0.06 a | 0.86 ± 0.12 bcd | |
| 3 | 59.40 ± 0.27 d | −1.49 ± 0.04 c | 26.11 ± 0.54 cd | 3.46 ± 0.11 bc | 3.44 ± 0.01 c | 11.63 ± 0.12 ab | 0.78 ± 0.06 d | |
| 7 | 59.77 ± 0.09 c | −1.46 ± 0.10 c | 25.69 ± 0.28 d | 3.43 ± 0.17 c | 3.44 ± 0.01 c | 11.63 ± 0.09 ab | 0.93 ± 0.06 a | |
| 14 | 59.63 ± 0.10 cd | −1.30 ± 0.04 b | 26.49 ± 0.31 c | 3.01 ± 0.10 d | 3.48 ± 0.01 b | 11.48 ± 0.13 b | 1.03 ± 0.13 abc | |
| 21 | 58.44 ± 0.14 e | −1.23 ± 0.12 ab | 27.59 ± 0.24 b | 3.73 ± 0.17 a | 3.48 ± 0.01 b | 11.56 ± 0.05 b | 1.09 ± 0.03 a | |
| 28 | 58.53 ± 0.06 e | −1.19 ± 0.05 ab | 27.62 ± 0.11 ab | 3.63 ± 0.07 ab | 3.50 ± 0.01 b | 11.78 ± 0.15 a | 1.06 ± 0.01 ab | |
| Sample | η = k ∙ γ(n−1) | G′ = K′ • ω n′ | G″ = K″ • ω n″ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | N | R2 | K′ | n′ | R2 | K″ | n″ | R2 | ||
| Orange juice | Control | 83.97 ± 4.66 | 0.37 ± 0.03 | 0.92 | 0.00 ± 0.00 | 2.08 ± 0.34 | 0.65 | 0.01 ± 0.00 | 2.08 ± 0.04 | 0.99 |
| 0 | 83.97 ± 4.66 | 0.37 ± 0.03 | 0.92 | 0.01 ± 0.00 | 2.08 ± 0.04 | 0.99 | 0.00 ± 0.00 | 2.07 ± 0.04 | 0.99 | |
| 28 | 42.3 ± 2.98 | 0.52 ± 0.04 | 0.74 | 0.00 ± 0.00 | 5.05 ± 1.53 | 0.42 | 0.00 ± 0.00 | 5.1 ± 0.08 | 1.00 | |
| Mixed juice | Control | 1960.29 ± 16.44 | 0.08 ± 0 | 1.00 | 2.24 ± 0.29 | 0.00 ± 0.00 | −0.02 | 0.02 ± 0.01 | 1.61 ± 0.09 | 0.95 |
| 0 | 1685.11 ± 10.32 | 0.00 ± 0.00 | 1.00 | 2.81 ± 0.37 | 0.00 ± 0.00 | −0.02 | 0.01 ± 0.01 | 1.91 ± 0.15 | 0.91 | |
| 28 | 3057.43 ± 39.43 | 0.17 ± 0.01 | 1.00 | 3.01 ± 0.49 | 0.00 ± 0.00 | −0.04 | 0.00 ± 0.00 | 4.15 ± 0.08 | 1.00 | |
| Kiwi-fruit puree | Control | 24520.41 ± 455.27 | 0.09 ± 0.01 | 1.00 | 56.83 ± 5.04 | 0.00 ± 0.00 | −0.02 | 0.06 ± 0.02 | 1.78 ± 0.06 | 0.98 |
| 0 | 24778.52 ± 364.16 | 0.12 ± 0.01 | 1.00 | 59.83 ± 6.04 | 0.00 ± 0.00 | 0.38 | 0.03 ± 0.02 | 1.64 ± 0.12 | 0.91 | |
| 28 | 28574.57 ± 285.76 | 0.18 ± 0.01 | 1.00 | 60.99 ± 5.62 | 0.00 ± 0.00 | −0.04 | 0.00 ± 0.00 | 3.04 ± 0.34 | 0.89 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, Y.; Jiang, Y.; Hu, X.; Yi, J. A Novel Strategy to Improve Cloud Stability of Orange-Based Juice: Combination of Natural Pectin Methylesterase Inhibitor and High-Pressure Processing. Foods 2023, 12, 581. https://doi.org/10.3390/foods12030581
Zhang W, Li Y, Jiang Y, Hu X, Yi J. A Novel Strategy to Improve Cloud Stability of Orange-Based Juice: Combination of Natural Pectin Methylesterase Inhibitor and High-Pressure Processing. Foods. 2023; 12(3):581. https://doi.org/10.3390/foods12030581
Chicago/Turabian StyleZhang, Wanzhen, Yantong Li, Yongli Jiang, Xiaosong Hu, and Junjie Yi. 2023. "A Novel Strategy to Improve Cloud Stability of Orange-Based Juice: Combination of Natural Pectin Methylesterase Inhibitor and High-Pressure Processing" Foods 12, no. 3: 581. https://doi.org/10.3390/foods12030581
APA StyleZhang, W., Li, Y., Jiang, Y., Hu, X., & Yi, J. (2023). A Novel Strategy to Improve Cloud Stability of Orange-Based Juice: Combination of Natural Pectin Methylesterase Inhibitor and High-Pressure Processing. Foods, 12(3), 581. https://doi.org/10.3390/foods12030581