Correlation between Water Characteristics and Gel Strength in the Gel Formation of Golden Pompano Surimi Induced by Dense Phase Carbon Dioxide
Abstract
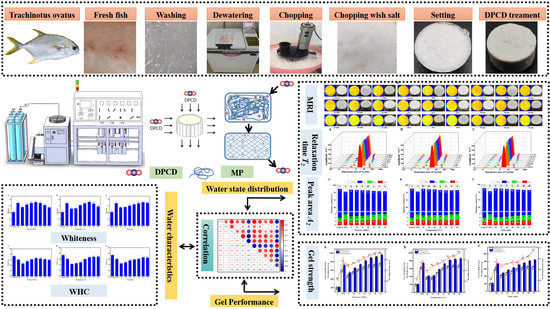
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Surimi Sample Preparation
2.3. Experimental Design
2.4. Determination of Whiteness
2.5. Determination of Water-Holding Capacity
2.6. Low-Field Nuclear Magnetic Resonance and Magnetic Resonance Imaging
2.7. Determination of Gel Strength
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different DPCD Treatments on Surimi Whiteness
3.2. Effect of Different DPCD Treatments on the Water-Holding Capacity of Surimi
3.3. Effect of Different DPCD Treatments on the Water Characteristics of Surimi
3.3.1. Effect of Different DPCD Treatments on the T2 Relaxation Times of Surimi
3.3.2. Effect of Different DPCD Treatments on the Different States of Water Content in Surimi
3.3.3. Effect of Different DPCD Treatments on Magnetic Resonance Imaging of Surimi
3.4. Effect of Different DPCD Treatments on the Surimi Gel Strength
3.5. Correlation between Water Characteristics and Gel Strength during DPCD-Induced Gel Formation in Surimi
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Guo, H.Y.; Zhu, K.C.; Guo, L.; Zhang, D.C. Growth, physiological, and molecular responses of golden pompano Trachinotus ovatus (linnaeus, 1758) reared at different salinities. Fish Physiol. Biochem. 2019, 45, 1879–1893. [Google Scholar] [CrossRef]
- Bureau of Fisheries of the Ministry of Agriculture. 2022 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2021; pp. 21–22.
- Wang, J.; Tang, J.; Park, J.W.; Rasco, B.; Liu, F.; Qu, Z. Thermal gelation of pacific whiting surimi in microwave assisted pasteurization. J. Food Eng. 2019, 258, 18–26. [Google Scholar] [CrossRef]
- Patil, U.; Saetang, J.; Zhang, B.; Benjakul, S. Use of tuna visceral pepsin in combination with trypsin as digestion aid: Enhanced protein hydrolysis and bioavailability. Foods 2023, 12, 125. [Google Scholar] [CrossRef]
- Zheng, O.; Sun, Q.; Dong, A.; Han, Z.; Wang, Z.; Wei, S.; Xia, Q.; Liu, Y.; Ji, H.; Liu, S. Gelation process optimization of shrimp surimi induced by dense phase carbon dioxide and quality evaluation of gel. Foods 2022, 11, 3807. [Google Scholar] [CrossRef]
- Rao, W.; Li, X.; Wang, Z.; Yang, Y.; Qu, Y.; Gao, Y.; Chen, L.; Zhang, D. Dense phase carbon dioxide combined with mild heating induced myosin denaturation, texture improvement and gel properties of sausage. J. Food Process Eng. 2017, 40, e12404. [Google Scholar] [CrossRef]
- Szerman, N.; Rao, W.; Li, X.; Yang, Y.; Vaudagna, S.R.; Zhang, D. Effects of the application of dense phase carbon dioxide treatments on technological parameters, physicochemical and textural properties and microbiological quality of lamb sausages. Food Eng. Rev. 2015, 7, 241–249. [Google Scholar] [CrossRef]
- Guo, M.; Liu, S.; Ismail, M.; Farid, M.M.; Ji, H.; Mao, W.; Gao, J.; Li, C. Changes in the myosin secondary structure and shrimp surimi gel strength induced by dense phase carbon dioxide. Food Chem. 2017, 227, 219–226. [Google Scholar] [CrossRef]
- Zhou, A.; Chen, H.; Zou, Y.; Liu, X.; Benjakul, S. Insight into the mechanism of optimal low-level pressure coupled with heat treatment to improve the gel properties of nemipterus virgatus surimi combined with water migration. Food Res. Int. 2022, 157, 111230. [Google Scholar] [CrossRef]
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined use of rheology, lf-nmr, and mri for characterizing the gel properties of hairtail surimi with potato starch. Food Bioprocess Technol. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Pan, Y.; Wei, S.; Xia, Q.; Liu, S.; Ji, H.; Deng, C.; Hao, J. Investigation on the correlation between changes in water and texture properties during the processing of surimi from golden pompano (Trachinotus ovatus). J. Food Sci. 2021, 86, 376–384. [Google Scholar] [CrossRef]
- Yang, Z.-f.; Liu, S.; Sun, Q.; Zheng, O.; Wei, S.; Xia, Q.; Ji, H.; Deng, C.; Hao, J.; Xu, J. Insight into muscle quality of golden pompano (Trachinotus ovatus) frozen with liquid nitrogen at different temperatures. Food Chem. 2021, 374, 131737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dong, X.-f.; Kong, B.; Sun, Q.; Ji, H.; Liu, S. Effects of magnetic field-assisted immersion freezing at different magnetic field intensities on the muscle quality of golden pompano (Trachinotus ovatus). Food Chem. 2023, 407, 135092. [Google Scholar] [CrossRef]
- Qian, Y.-F.; Xie, J.; Yang, S.P.; Wu, W.-H. Study of the quality changes and myofibrillar proteins of white shrimp (litopenaeus vannamei) under modified atmosphere packaging with varying CO2 levels. Eur. Food Res. Technol. 2013, 236, 629–635. [Google Scholar] [CrossRef]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Influence of high pressure on the color and microbial quality of beef meat. LWT—Food Sci. Technol. 2003, 36, 625–631. [Google Scholar] [CrossRef]
- Cando, D.; Herranz, B.; Borderías, A.J.; Moreno, H.M. Effect of high pressure on reduced sodium chloride surimi gels. Food Hydrocoll. 2015, 51, 176–187. [Google Scholar] [CrossRef]
- Moreno, H.M.; Bargiela, V.; Tovar, C.A.; Cando, D.; Borderías, A.J.; Herranz, B. High pressure applied to frozen flying fish (Parexocoetus brachyterus) surimi: Effect on physicochemical and rheological properties of gels. Food Hydrocoll. 2015, 48, 127–134. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, L.; Jiang, X.; Chen, Y.; Zhou, G.-H. The effects of three polysaccharides on the gelation properties of myofibrillar protein: Phase behaviour and moisture stability. Meat Sci. 2020, 170, 108228. [Google Scholar] [CrossRef]
- Yuan, L.; Dang, Q.-L.; Mu, J.; Feng, X.; Gao, R. Mobility and redistribution of waters within bighead carp (Aristichthys nobilis) heat-induced myosin gels. Int. J. Food Prop. 2018, 21, 835–849. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Tashiro, Y.; Ogawa, H. Relationship between the water molecules in fish-meat gel and the gel structure. Fish. Sci. 2012, 78, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Tashiro, Y.; Matsukawa, S.; Ogawa, H. Gelation mechanism of surimi studied by 1H NMR relaxation measurements. J. Food Sci. 2007, 72, E362–E367. [Google Scholar] [CrossRef]
- Srikaeo, K.; Rahman, M.S. Proton relaxation of waxy and non-waxy rice by low field nuclear magnetic resonance (lf-nmr) to their glassy and rubbery states. J. Cereal Sci. 2018, 82, 94–98. [Google Scholar] [CrossRef]
- Lan, W.; Zhao, Y.; Gong, T.; Mei, J.; Xie, J. Effects of different thawing methods on the physicochemical changes, water migration and protein characteristic of frozen pompano (Trachinotus ovatus). J. Food Biochem. 2021, 45, e13826. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Zhu, Y.; Ye, T.; Nie, Y.F.; Jiang, S.; Lin, L.; Lu, J. Physicochemical properties and microstructure of composite surimi gels: The effects of ultrasonic treatment and olive oil concentration. Ultrason. Sonochem. 2022, 88, 106065. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Di, S.; Liu, D.; Sun, J.; Shao, J.-H. Low-field NMR and FTIR determination relationship between water migration and protein conformation of the preparation of minced meat. Int. J. Food Sci. Technol. 2022, 57, 235–241. [Google Scholar] [CrossRef]
- Kustyawati, M.E.; Pratama, F.; Rizal, S.; Fadhallah, E.G.; Damai, A.A. Quality and shelf life of white shrimp (Litopenaeus vannamei) processed with high-pressure carbon dioxide (hpcd) at subcritical and supercritical states. J. Food Qual. 2021, 1, 6649583. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, L.; Luo, S.; Dong, S.; Mengna, L.; Ji, H.; Gao, J.; Hao, J. Molecular dynamics simulation of the interaction between dense-phase carbon dioxide and the myosin heavy chain. J. CO2 Util. 2017, 21, 270–279. [Google Scholar] [CrossRef]
- Sang, S.; Chen, X.; Qin, Y.; Tong, L.; Ou, C. A study on the effects of calcium lactate on the gelling properties of large yellow croaker (Pseudosciaena crocea) surimi by low-field nuclear magnetic resonance and raman spectroscopy. Foods 2022, 11, 3197. [Google Scholar] [CrossRef]
- Ezeanaka, M.C.; Nsor-Atindana, J.; Zhang, M. Online low-field nuclear magnetic resonance (lf-nmr) and magnetic resonance imaging (mri) for food quality optimization in food processing. Food Bioprocess Technol. 2019, 12, 1435–1451. [Google Scholar] [CrossRef]
- Gao, Y.; Fukushima, H.; Deng, S.-G.; Jia, R.; Osako, K.; Okazaki, E. Effect of emulsifying stability of myofibrillar protein on the gel properties of emulsified surimi gel. Food Sci. Nutr. 2018, 6, 1229–1237. [Google Scholar] [CrossRef]
- Illera, A.E.; Sanz, M.T.; Beltrán, S.; Benito-Román, Ó. Effect of high pressure carbon dioxide on polyphenoloxidase from litopenaeus vannamei. LWT—Food Sci. Technol. 2019, 109, 359–365. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Zhou, A.; Xiao, J.; Benjakul, S. Insight into the effect of ice addition on the gel properties of nemipterus virgatus surimi gel combined with water migration. Foods 2021, 10, 1815. [Google Scholar] [CrossRef] [PubMed]
- Huggins, D.J. Studying the role of cooperative hydration in stabilizing folded protein states. J. Struct. Biol. 2016, 196, 394–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballauff, M. Denaturation of proteins: Electrostatic effects vs. Hydration. RSC Adv. 2022, 12, 10105–10113. [Google Scholar] [CrossRef] [PubMed]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, W.; Qiu, H.; Htwe, K.K.; Wang, Z.; Liu, Y.; Wei, S.; Xia, Q.; Sun, Q.; Han, Z.; Liu, S. Correlation between Water Characteristics and Gel Strength in the Gel Formation of Golden Pompano Surimi Induced by Dense Phase Carbon Dioxide. Foods 2023, 12, 1090. https://doi.org/10.3390/foods12051090
Duan W, Qiu H, Htwe KK, Wang Z, Liu Y, Wei S, Xia Q, Sun Q, Han Z, Liu S. Correlation between Water Characteristics and Gel Strength in the Gel Formation of Golden Pompano Surimi Induced by Dense Phase Carbon Dioxide. Foods. 2023; 12(5):1090. https://doi.org/10.3390/foods12051090
Chicago/Turabian StyleDuan, Weiwen, Hui Qiu, Kyi Kyi Htwe, Zefu Wang, Yang Liu, Shuai Wei, Qiuyu Xia, Qinxiu Sun, Zongyuan Han, and Shucheng Liu. 2023. "Correlation between Water Characteristics and Gel Strength in the Gel Formation of Golden Pompano Surimi Induced by Dense Phase Carbon Dioxide" Foods 12, no. 5: 1090. https://doi.org/10.3390/foods12051090
APA StyleDuan, W., Qiu, H., Htwe, K. K., Wang, Z., Liu, Y., Wei, S., Xia, Q., Sun, Q., Han, Z., & Liu, S. (2023). Correlation between Water Characteristics and Gel Strength in the Gel Formation of Golden Pompano Surimi Induced by Dense Phase Carbon Dioxide. Foods, 12(5), 1090. https://doi.org/10.3390/foods12051090