Inhibition of Browning in Apples Using Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green Rooibos and Reagents
2.2. Solid–Liquid Extraction of Green Rooibos and Analysis of Bioactive Compounds
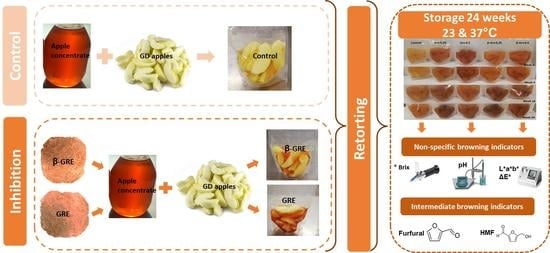
2.3. The Canning Process
2.4. Sample Preparation
2.5. Browning Indices Colour Measurement (L, a*, b*)
2.6. Determination of 5-hydroxymethyl Furfural and Furfural
2.7. Statistical Analysis
3. Results and Discussion
3.1. Inhibition of Colour Formation Via L* Value and DE*
3.2. Inhibition of Furfural and HMF Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burdurlu, H.S.; Karadeniz, F. Effect of storage on non-enzymatic browning of apple juice concentrates. Food Chem. 2003, 80, 91–97. [Google Scholar] [CrossRef]
- Paravisini, L.; Peterson, D.G. Role of Reactive Carbonyl Species in non-enzymatic browning of apple juice during storage. Food Chem. 2018, 245, 1010–1017. [Google Scholar] [CrossRef]
- Aktağ, G.; Gökmen, V. Multiresponse kinetic modelling of α-dicarbonyl compounds formation in fruit juices during storage. Food Chem. 2020, 320, 126620. [Google Scholar] [CrossRef]
- Aktag, G.; Gokmen, V. Investigations on the formation of α -dicarbonyl compounds and 5-hydroxymethylfurfural in fruit products during storage: New insights into the role of Maillard reaction. Food Chem. 2021, 363, 130280. [Google Scholar] [CrossRef] [PubMed]
- Garza, S.; Ibarz, A.; Paga, J. Kinetic models of non-enzymatic browning in apple puree. J. Sci. Food Agric. 2000, 80, 1162–1168. [Google Scholar]
- Louarme, L.; Billaud, C. Evaluation of ascorbic acid and sugar degradation products during fruit dessert processing under conventional or ohmic heating treatment. LWT-Food Sci. Technol. 2012, 49, 184–187. [Google Scholar] [CrossRef]
- Herbig, A.L.; Renard, C.M.G.C. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef]
- Buvé, C.; Pham, H.T.T.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Reaction pathways and factors influencing non-enzymatic browning in shelf-stable fruit juices during storage. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5698–5721. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B. Non-enzymatic browning in citrus juice: Chemical markers, their detection and ways to improve product quality. J. Food Sci. Technol. 2014, 51, 2271–2288. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef] [PubMed]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Zhong, Y.J.; Perera, N.; Shahidi, F. Antiglycation activity of lipophilized epigallocatechin gallate (EGCG) derivatives. Food Chem. 2016, 190, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.; de Oliveira, M.R.; da Veiga Pereira, T.; Reginatto, F.H.; Dal-Pizzol, F.; Moreira, J.C.F. Antioxidant and antiglycation properties of Passiflora alata and Passiflora edulis extracts. Food Chem. 2007, 100, 719–724. [Google Scholar] [CrossRef]
- Wu, J.W.; Hsieh, C.L.; Wang, H.Y.; Chen, H.Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Hafsa, J.; Hammi, K.M.; Le Cerf, D.; Limem, K.; Majdoub, H.; Charfeddine, B. Characterisation, antioxidant and antiglycation properties of polysaccharides extracted from the medicinal halophyte Carpobrotus edulis L. Int. J. Biol. Macromol. 2018, 107, 833–842. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W. Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]
- Abrantes, T.; Moura-Nunes, N.; Perrone, D. Gallic Acid Mitigates 5-Hydroxymethylfurfural Formation while Enhancing or Preserving Browning and Antioxidant Activity Development in Glucose/Arginine and Sucrose/Arginine Maillard Model Systems. Molecules 2022, 27, 848. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; DeBeer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Miller, N. Green Rooibos Nutraceutical: Optimisation of Hot Water Extraction and Spray-Drying by Quality-by-Design Methodology. Ph.D. Dissertation, University of Stellenbosch, Stellenbosch, South Africa, 2016. Available online: http://hdl.handle.net/10019.1/100264 (accessed on 18 July 2022).
- Damiani, E.; Carloni, P.; Rocchetti, G.; Senizza, B.; Tiano, L.; Joubert, E.; de Beer, D.; Lucini, L. Impact of Cold versus Hot Brewing on the Phenolic Profile and Antioxidant Capacity of Rooibos (Aspalathus linearis) Herbal Tea. Antioxidants 2019, 8, 499. [Google Scholar] [CrossRef]
- Joubert, E.; DeBeer, D. Antioxidants of rooibos beverages: Role of plant composition and processing. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Elsevier Academic Press: Woburn, MA, USA, 2014; pp. 131–144. [Google Scholar]
- Favreau-Farhadi, N.; Pecukonis, L.; Barrett, A. The Inhibition of Maillard Browning by Different Concentrations of Rosmarinic Acid and Epigallocatechin-3-Gallate in Model, Bakery, and Fruit Systems. J. Food Sci. 2015, 80, 2140–2146. [Google Scholar] [CrossRef]
- Albahari, P.; Jug, M.; Radić, K.; Jurmanović, S.; Brnčić, M.; Brnčić, S.R.; Čepo, D.V. Characterisation of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT-Food Sci. Technol. 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Li, D.; Zhu, M.; Liu, X.; Wang, Y.; Cheng, J. Insight into the effect of microcapsule technology on the processing stability of mulberry polyphenols. LWT-Food Sci. Technol. 2020, 126, 109144. [Google Scholar] [CrossRef]
- Human, C.; de Beer, D.; Aucamp, M.; Marx, I.J.; Malherbe, C.J.; Viljoen-Bloom, M.; van der Rijst, M.; Joubert, E. Preparation of rooibos extract-chitosan microparticles: Physicochemical characterisation and stability of aspalathin during accelerated storage. LWT-Food Sci. Technol. 2020, 117, 108653. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.M. Room at the Top as well as at the Bottom: Structure of Functional Food Inclusion Compounds. In Cyclodextrin—A Versatile Ingredient; Arora, P., Dhingra, N., Eds.; Intechopen: London, UK, 2018; pp. 119–134. [Google Scholar]
- Lavelli, V.; Harsha, P.S.C.S. Microencapsulation of grape skin phenolics for pH controlled release of antiglycation agents. Food Res. Int. 2019, 119, 822–828. [Google Scholar] [CrossRef]
- Favre, L.C.; Rolandelli, G.; Mshicileli, N.; Vhangani, L.N.; dos Santos Ferreira, C.; van Wyk, J.; del Pilar Buera, M. Antioxidant and anti-glycation potential of green pepper (Piper nigrum): Optimisation of β-cyclodextrin-based extraction by response surface methodology. Food Chem. 2020, 316, 126280. [Google Scholar] [CrossRef]
- Maraulo, G.E.; Ferreira, C.S.; Mazzobre, M.F. Β-Cyclodextrin Enhanced Ultrasound-Assisted Extraction As a Green Method To Recover Olive Pomace Bioactive Compounds. J. Food Process. Preserv. 2021, 45, e15194. [Google Scholar] [CrossRef]
- Favre, L.C.; Santos, C.; López-Fernández, M.P.; Mazzobre, M.F.; del Pilar Buera, M. Optimisation of β-cyclodextrin-based extraction of antioxidant and anti-browning activities from thyme leaves by response surface methodology. Food Chem. 2018, 265, 86–95. [Google Scholar] [CrossRef]
- El Darra, N.; El Darra, N.; Rajha, H.N.; Saleh, F.; El-Ghazzawi, I.; Louka, N.; Maroun, R.G. Comparative Study between Ethanolic and Cyclodextrin Assisted Extraction of Polyphenols from Peach Pomace. In Proceedings of the 3rd ISANH Middle East Antioxidants World Congress, Royal Scientific Society, Amman, Jordan, 2–3 May 2018. [Google Scholar]
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends Food Sci. Technol. 2018, 79, 19–27. [Google Scholar] [CrossRef]
- Anonymous. Agricultural Product Standard Act No 119 of 1990. Regulation Relating to the Grading, Packing and Marking of Canned Fruit Intended for Sale in the Republic of South Africa; South African Government: Pretoria, South Africa, 2022. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/272800.pdf (accessed on 12 October 2022).
- Ghoul, I.I.M. Prevention of Enzymatic Browning in Fruits and Vegetables. Eur. Sci. J. 2013, 9, 310–341. [Google Scholar]
- Liao, H.; Zhu, W.; Zhong, K.; Liu, Y. Evaluation of colour stability of clear red pitaya juice treated by thermosonication. LWT-Food Sci. Technol. 2020, 121, 108997. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Wu, G.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. Reduction of 5-hydroxymethylfurfural formation by flavan-3-ols in Maillard reaction models and fried potato chips. J. Sci. Food Agric. 2018, 98, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Liu, X.; Bi, J.; Wu, X.; Zhou, L.; Ruan, W. Kinetic modelling of non-enzymatic browning and changes of physio-chemical parameters of peach juice during storage. J. Food Sci. Technol. 2018, 55, 1003–1009. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Favre, L.C.; Rolandelli, G.; Van Wyk, J.; del Pilar Buera, M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules 2022, 27, 3556. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, A.; Pag, J.; Garza, S. Kinetic models for colour changes in pear puree during heating at relatively high temperatures. J. Food Eng. 1999, 39, 415–422. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey Colour difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Oral, R.A.; Dogan, M.; Sarioglu, K. Effects of certain polyphenols and extracts on furans and acrylamide formation in a model system, and total furans during storage. Food Chem. 2014, 142, 423–429. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Kinetics of heat-induced changes in foods: A workflow proposal. J. Food Eng. 2021, 306, 110634. [Google Scholar] [CrossRef]

| Sample Type | Lightness Value (L*) | Colour Difference (DE*) | |||||
|---|---|---|---|---|---|---|---|
| Conc [] | Temp ( °C) | % IL* | (k0) | r2 | % IDE* | (k0) | r2 |
| Control | 23 | - | −0.9786 ± 0.38 d | 0.9286 | - | 1.0205 ± 0.69 b | 0.9377 |
| Gre 0.25 | 23 | 18.45 ± 3.64 c | −0.7536 ± 0.39 bc | 0.9575 | 8.94± 0.99 b | 0.8984 ± 1.09 b | 0.9582 |
| Gre 0.5 | 23 | 17.07 ± 1.93 c | −0.8283 ± 0.17 c | 0.9454 | 21.23 ± 1.20 c | 0.8555± 1.91 b | 0.9546 |
| β-Gre 0.25 | 23 | 40.93 ± 2.71 d | −0.6620 ± 0.55 b | 0.6918 | 36.22 ± 4.33 d | 0.6551 ± 1.32 a | 0.6803 |
| β-Gre 0.5 | 23 | 46.67 ± 5.96 e | −0.5325 ± 0.81 a | 0.8946 | 45.24 ± 3.06 e | 0.6106 ± 0.23 a | 0.8957 |
| Control | 37 | - | −1.1130 ±1.27 C | 0.9670 | - | 1.0785± 0.51 C | 0.9465 |
| Gre 0.25 | 37 | 18.55 ± 2.44 c | −0.9199 ± 1.42 A | 0.9730 | 9.68 ± 1.46 b | 0.9954 ± 1.00 BC | 0.9321 |
| Gre 0.5 | 37 | 0.20 ± 0.98 a | −0.9179 ± 1.83 A | 0.9705 | 9.54 ± 1.3 b | 0.9262 ± 2.04 AB | 0.9582 |
| β-Gre 0.25 | 37 | 9.82 ± 1.68 b | −0.9210 ± 0.43 A | 0.9208 | 11.96 ± 2.15 b | 0.8657 ± 1.42 A | 0.9060 |
| β-Gre 0.5 | 37 | 2.83 ± 0.83 a | −1.0361± 0.744 B | 0.9622 | −17.17 ± 6.51 a | 1.0929 ± 0.47 C | 0.9454 |
| Sample Type | Furfural | HMF | |||||
|---|---|---|---|---|---|---|---|
| Conc [] | Temp ( °C) | %IF | (k0) | r2 | %Inhibition | (k0) | r2 |
| Control | 23 | - | 0.1241 ± 0.55 b | 0.7787 | - | 0.0865 ± 0.85 d | 0.9511 |
| Gre 0.25 | 23 | 62.69 ± 1.46 c | 0.0320 ± 0.69 a | 0.7081 | 12.75 ± 12.75 a | 0.0746 ± 0.14 c | 0.9297 |
| Gre 0.5 | 23 | 64.55 ± 4.16 c | 0.0379 ± 0.24 a | 0.8948 | 50.20 ± 3.56 d | 0.0459 ± 0.60 b | 0.9567 |
| β-Gre 0.25 | 23 | 72.29 ± 1.20 c | 0.0322 ± 0.12 a | 0.8688 | 59.55 ± 5.64 e | 0.0273 ± 0.46 a | 0.8002 |
| β-Gre 0.5 | 23 | 63.54 ± 9.57 c | 0.0376 ± 0.17 a | 0.8676 | 67.33 ± 5.22 e | 0.0301 ± 0.47 a | 0.9121 |
| Control | 37 | - | 0.8657± 1.42 B | 0.9388 | - | 0.2124 ± 0.08 D | 0.9751 |
| Gre 0.25 | 37 | 10.17 ± 1.34 a | 0.7160 ± 1.6 AB | 0.8802 | 11.51 ± 1.21 a | 0.1774 ± 0.08 C | 0.8998 |
| Gre 0.5 | 37 | 47.10 ± 1.51 b | 0.4892 ± 0.47 A | 0.9414 | 24.03 ± 8.39 b | 0.1573 ± 0.11 B | 0.9274 |
| β-Gre 0.25 | 37 | 35.04 ± 3.09 b | 0.5275 ± 0.77 A | 0.8763 | 36.56 ± 3.14 c | 0.1215 ± 0.04 A | 0.8797 |
| β-Gre 0.5 | 37 | 43.87 ± 1.51 b | 0.4877 ± 0.69 A | 0.9485 | 45.77 ± 2.62 cd | 0.1143 ± 0.61 A | 0.9518 |
| Browning Development | Intermediate Products | |||
|---|---|---|---|---|
| Sample type | L* Value | DE* | HMF | Furfural |
| Control | 7.03 ± 1.79 a | 3.16 ± 3.33 a | 49.12 ± 7.12 a | 101.88 ± 3.53 a |
| Gre 0.25 | 10.83 ± 2.15 ab | 5.87 ± 4.70 a | 47.23 ± 3.53 a | 167.13 ± 5.34 d |
| Gre 0.5 | 5.55 ± 1.72 a | 4.42 ± 2.31 a | 67.42 ± 3.10 b | 140.06 ± 1.83 b |
| β-Gre 0.25 | 18.84 ± 10.28 b | 15.54 ± 3.64 b | 81.92 ± 5.64 c | 148.82 ± 3.54 c |
| β-Gre 0.5 | 36.33 ± 1.39 c | 32.06 ± 7.47 c | 73.12 ± 8.54 bc | 136.79 ± 3.63 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vhangani, L.N.; Van Wyk, J. Inhibition of Browning in Apples Using Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis). Foods 2023, 12, 602. https://doi.org/10.3390/foods12030602
Vhangani LN, Van Wyk J. Inhibition of Browning in Apples Using Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis). Foods. 2023; 12(3):602. https://doi.org/10.3390/foods12030602
Chicago/Turabian StyleVhangani, Lusani Norah, and Jessy Van Wyk. 2023. "Inhibition of Browning in Apples Using Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis)" Foods 12, no. 3: 602. https://doi.org/10.3390/foods12030602
APA StyleVhangani, L. N., & Van Wyk, J. (2023). Inhibition of Browning in Apples Using Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis). Foods, 12(3), 602. https://doi.org/10.3390/foods12030602