Total Phenolic Content and Antioxidant Activity of In Vitro Digested Hemp-Based Products
Abstract
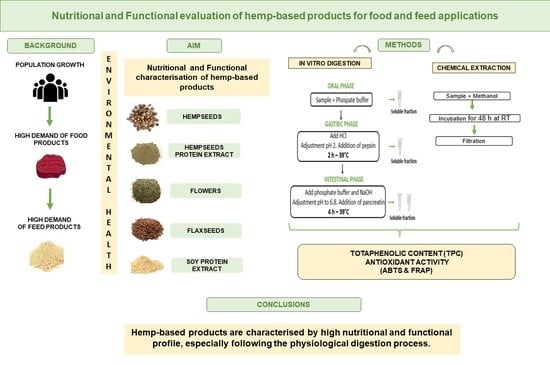
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Analysis
2.3. Methanol Extraction
2.4. Total Phenolic Content and Antioxidant Activity
2.4.1. Total Phenolic Content
2.4.2. ABTS Assay
2.4.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.5. In Vitro Digestion and Digestibility
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis and In Vitro Digestibility
3.2. Total Phenolic Content and Antioxidant Activity of Methanol Extracts
3.2.1. Total Phenolic Content of Methanol Extracts
3.2.2. Antioxidant Activity of Methanol Extracts
3.3. Total Phenolic Content and Antioxidant Activity of In Vitro Digested Samples
3.3.1. Total Phenolic Content of In Vitro Digested Samples
3.3.2. Antioxidant Activity of In Vitro Digested Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Röös, E.; Bajželj, B.; Smith, P.; Patel, M.; Little, D.; Garnett, T. Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob. Environ. Chang. 2017, 47, 1–12. [Google Scholar] [CrossRef]
- Bellet, C.; Rushton, J. World food security, globalisation and animal farming: Unlocking dominant paradigms of animal health science. Rev. Off. Int. Epizoot 2019, 38, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Rischer, H.; Szilvay, G.R.; Oksman-Caldentey, K.M. Cellular agriculture—Industrial biotechnology for food and materials. Curr. Opin. Biotechnol. 2020, 61, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H. Review: Feed demand landscape and implications of food-not feed strategy for food security and climate change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Teitge, J.; Mielke, J.; Schütze, F.; Jaeger, C. The European Green Deal—More Than Climate Neutrality. Intereconomics 2021, 56, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.R. Importance of a One Health approach in advancing global health security and the Sustainable Development Goals. Rev. Off. Int. Epizoot 2019, 38, 145–154. [Google Scholar] [CrossRef]
- Rehman, M.; Fahad, S.; Du, G.; Cheng, X.; Yang, Y.; Tang, K.; Liu, L.; Liu, F.H.; Deng, G. Evaluation of hemp (Cannabis sativa L.) as an industrial crop: A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 52832–52843. [Google Scholar] [CrossRef]
- Petit, J.; Salentijn, E.M.J.; Paulo, M.J.; Thouminot, C.; van Dinter, B.J.; Magagnini, G.; Gusovius, H.J.; Tang, K.; Amaducci, S.; Wang, S.; et al. Genetic Variability of Morphological, Flowering, and Biomass Quality Traits in Hemp (Cannabis sativa L.). Front. Plant. Sci. 2020, 20, 102. [Google Scholar] [CrossRef]
- Sorrentino, G. Introduction to emerging industrial applications of cannabis (Cannabis sativa L.). Rend. Lincei. Sci. Fis. E Nat. 2021, 32, 233–243. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety of hemp (Cannabis genus) for use as animal feed. EFSA J. 2011, 9, 2011. [Google Scholar]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 7, 4078. [Google Scholar] [CrossRef] [PubMed]
- Guang, H.; Wenwei, C. Application of Powder of Whole Cannabis Sativa Seeds for Preparing Functional Food with Adjuvant Therapy of Lowering Blood Fat. China Patent No. 100998414 B, 2010. Available online: https://patents.google.com/patent/CN100998414B/en (accessed on 12 May 2020).
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- European Commission, Commission Regulation (EC). No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed establishes the sampling method and the methods of analysis of feed for control purposes. European Union. Off. J. Eur. Union 2009, 54, 1–130. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Regmi, P.R.; Ferguson, N.S.; Zijlstra, R.T. In vitro digestibility techniques to predict apparent total tract energy digestibility of wheat in grower pigs. J. Anim. Sci. 2009, 87, 3620–3629. [Google Scholar] [CrossRef]
- Abdelaleem, M.A.; Elbassiony, K. Evaluation of phytochemicals and antioxidant activity of gamma irradiated quinoa (Chenopodium quinoa). Braz. J. Biol. 2021, 81, 806–813. [Google Scholar] [CrossRef]
- Lan, Y.; Zha, F.; Peckrul, A.; Hanson, B.; Johnson, B.; Rao, J.; Chen, B. Genotype x Environmental Effects on Yielding Ability and Seed Chemical Composition of Industrial Hemp (Cannabis sativa L.) Varieties Grown in North Dakota, USA. J. Am. Oil Chem. Soc. 2019, 96, 1417–1425. [Google Scholar] [CrossRef]
- Mattila, P.; Pihlava, J.M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282–308. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Zając, M.; Kiczorowska, B.; Samolińska, W.; Kowalczyk-Pecka, D.; Andrejko, D.; Kiczorowski, P. Effect of inclusion of micronized camelina, sunflower, and flax seeds in the broiler chicken diet on performance productivity, nutrient utilization, and intestinal microbial populations. Poult. Sci. 2021, 100, 101118. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A potential source of food, feed and fiber. Crit. Rev. Food Sci. Nutr. 2011, 51, 210–222. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Huang, F.; Yang, C.; Huang, Q. In Vitro Digestion and Fermentation by Human Fecal Microbiota of Polysaccharides from Flaxseed. Molecules 2020, 25, 4354. [Google Scholar] [CrossRef]
- Marambe, H.K.; Shand, P.J.; Wanasundara, J.P. In vitro digestibility of flaxseed (Linum usitatissimum L.) protein: Effect of seed mucilage, oil and thermal processing. Int. J. Food Sci. Technol. 2013, 48, 628–635. [Google Scholar] [CrossRef]
- Kleinhenz, M.D.; Magnin, G.; Ensley, S.M.; Griffin, J.J.; Goeser, J.; Lynch, E.; Coetzee, J.F. Nutrient concentrations, digestibility, and cannabinoid concentrations of industrial hemp plant components. Appl. Anim. Sci. 2020, 36, 489–494. [Google Scholar] [CrossRef]
- Mamone, G.; Picariello, G.; Ramondo, A.; Nicolai, M.A.; Ferranti, P. Production, digestibility and allergenicity of hemp (Cannabis sativa L.) protein isolates. Food Res. Int. 2019, 115, 562–571. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Yang, X.Q.; Gao, W.R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Tang, C.H.; Wang, X.S.; Yang, X.Q. Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chem. 2009, 114, 1484–1490. [Google Scholar] [CrossRef]
- Kalinowska, M.; Płońska, A.; Trusiak, M.; Gołębiewska, E.; Gorlewska-Pietluszenko, A. Comparing the extraction methods, chemical composition, phenolic contents and antioxidant activity of edible oils from Cannabis sativa and Silybum marianu seeds. Sci. Rep. 2022, 12, 20609. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Chang, S.K.; Bolling, B.; Oh, W.Y.; Shahidi, F. Specialty seeds: Nutrients, bioactives, bioavailability, and health benefits: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2382–2427. [Google Scholar] [CrossRef] [PubMed]
- Galasso, I.; Russo, R.; Mapelli, S.; Ponzoni, E.; Brambilla, I.M.; Battelli, G.; Reggiani, R. Variability in Seed Traits in a Collection of Cannabis sativa L. Genotypes. Front. Plant Sci. 2016, 7, 688. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.M.; Hellström, J.; Pihlanto, A. Nutritional Value of Commercial Protein-Rich Plant Products. Plant Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Sakač, M.; Dapčević Hadnađev, T.; Šarić, B.; Milovanović, I.; Hadnađev, M. Characterization of byproducts originating from hemp oil processing. J. Agric. Food Chem. 2014, 62, 12436–12442. [Google Scholar] [CrossRef]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic Compounds and Antioxidant Activity of Cold-Pressed Seed Oil from Finola Cultivar of Cannabis sativa L. Phytother. Res. 2016, 30, 1298–1307. [Google Scholar] [CrossRef]
- Bourjot, M.; Zedet, A.; Demange, B.; Pudlo, M.; Girard-Thernier, C. In Vitro Mammalian Arginase Inhibitory and Antioxidant Effects of Amide Derivatives Isolated from the Hempseed Cakes (Cannabis sativa). Planta Med. Int. Open 2017, 3, e64–e67. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [Green Version]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postepy High Med. Dosw. 2013, 67, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Peres, F.F.; Lima, A.C.; Hallak, J.E.C.; Crippa, J.A.; Silva, R.H.; Abílio, V.C. Cannabidiol as a Promising Strategy to Treat and Prevent Movement Disorders? Front. Pharmacol. 2018, 9, 482. [Google Scholar] [CrossRef]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef]
- Giromini, C.; Tretola, M.; Baldi, A.; Ottoboni, M.; Rebucci, R.; Manoni, M.; Di Lorenzo, C.; Pinotti, L. Total phenolic content and antioxidant capacity of former food products intended as alternative feed ingredients. Ital. J. Anim. Sci. 2020, 19, 1387–1392. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Gao, J.; Feng, J.; Shang, Y.; Wei, Z. Phenolics and antioxidant activity of bamboo leaves soup as affected by in vitro digestion. Food Chem. Toxicol. 2020, 135, 110941. [Google Scholar] [CrossRef]
- Goulas, V.; Hadjisolomou, A. Dynamic changes in targeted phenolic compounds and antioxidant potency of carob fruit (Ceratonia siliqua L.) products during in vitro digestion. Food Sci. Technol. 2019, 101, 269–275. [Google Scholar] [CrossRef]
- Vinholes, J.; Reis, S.F.; Lemos, G.; Barbieri, R.L.; de Freitas, V.; Franzon, R.C.; Vizzotto, M. Effect of in vitro digestion on the functional properties of Psidium cattleianum Sabine (araçá), Butia odorata (Barb. Rodr.) Noblick (butiá) and Eugenia uniflora L. (pitanga) fruit extracts. Food Func. 2018, 9, 6380–6390. [Google Scholar] [CrossRef]
- Ginsburg, I.; Koren, E.; Shalish, M.; Kanner, J.; Kohen, R. Saliva increases the availability of lipophilic polyphenols as antioxidants and enhances their retention in the oral cavity. Arch. Oral Biol. 2012, 57, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Func. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Giromini, C.; Cavalleri, M. Bioactive Peptides: Cytomodulatory Activity. In Bioactive Peptides from Food; CRC Press: Boca Raton, FL, USA, 2022; pp. 461–484. [Google Scholar]
- Teh, S.S.; Bekhit, A.; Carne, A.; Birch, J. Antioxidant and ACE-inhibitory activities of hemp (Cannabis sativa L.) protein hydrolysates produced by the proteases AFP, HT, Pro-G, actinidin and zingibain. Food Chem. 2016, 203, 199–206. [Google Scholar] [CrossRef]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Hempseed Peptides Exert Hypocholesterolemic Effects with a Statin-Like Mechanism. J. Agric. Food Chem. 2017, 65, 8829–8838. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S.A.; Aluko, R.E. In vitro acetylcholinesterase-inhibitory properties of enzymatic hemp seed protein hydrolysates. J. Am. Oil Chem. 2016, 93, 411–420. [Google Scholar] [CrossRef]
- Tang, C.H.; Ten, Z.; Wang, X.S.; Yang, X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Chen, L.; Yang, X.Q. Characterization and antioxidant properties of hemp protein hydrolysates obtained with Neutrase®. Food Technol. Biotechnol. 2009, 47, 428–434. [Google Scholar]
- Koocheki, A.; Hesarinejad, M.A.; Mozafari, M.R. Lepidium perfoliatum seed gum: Investigation of monosaccharide composition, antioxidant activity and rheological behavior in presence of salts. Chem. Biol. Technol. Agric. 2022, 9, 61. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez Álvarez, J.A.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef] [PubMed]
- WWF. 2017. Available online: http://wwf.panda.org/what_we_do/footprint/agriculture/soy/facts/ (accessed on 21 October 2017).
- Eriksson, M.; Ghosh, R.; Hansson, E.; Basnet, S.; Lagerkvist, C.J. Environmental consequences of introducing genetically modified soy feed in Sweden. J. Clean. 2018, 176, 46–53. [Google Scholar] [CrossRef]


| SAMPLE | DM | CP | EE | NDF | ADF | ADL | ASHES | DIGESTIBILITY |
|---|---|---|---|---|---|---|---|---|
| Hempseeds | 94.6 ± 0.12 a | 23.1 ± 0.57 a | 27.9 ± 0.75 a | 44.6 ± 0,21 a | 33.2 ± 0.31 a | 14.4 ± 0.32 a | 5.8 ± 0.10 a | 53.4 ± 0.78 a |
| Flowers | 98.4 ± 0.03 b | 18.9 ± 0.29 b | 12.9 ± 0.25 b | 37.0 ± 0,67 b | 23.7 ± 0.35 b | 11.7 ± 0.79 b | 16.3 ± 0.08 b | 37.4 ± 1.87 b |
| Hempseed protein Extract | 95.7 ± 0.02 c | 45.1 ± 0.84 c | 9.2 ± 0.09 c | 40.4 ± 1.80 a,b | 20.2 ± 1.02 c | 9.5 ± 0.62 b,d | 8.3 ± 0.08 c | 65.5 ± 0.67 c |
| Soy protein extract | 91.1 ± 0.22 d | 51.2 ± 0.57 d | 1.2 ± 0.06 d | 48.2 ± 0.97 a,c | 8.8 ± 0.14 d | 2.2 ± 0.10 c | 6.6 ± 0.20 d | 74.1 ± 3.59 c |
| Flaxseeds | 91.6 ± 0.14 d | 23.0 ± 0.45 a | 36.0 ± 0.07 e | 41.5 ± 1.27 a,b | 19.5 ± 0.35 c | 9.2 ± 0.41 d | 2.7 ± 0.20 e | 24.2 ± 2.08 d |
| SAMPLE | TPC (mg TAE/100 g) | ABTS (mg TE/100 g) | FRAP (mg AAE/100 g) |
|---|---|---|---|
| Hempseeds | 550.3 ± 28.27 a | 205.4 ± 3.37 a | 50.9 ± 4.30 a |
| Flowers | 2982.8 ± 167.78 b | 6122.1 ± 249.52 b | 123.6 ± 8.08 b |
| Hempseed protein extract | 568.9 ± 34.18 a | 174.5 ± 4.30 a | 29.73 ± 1.32 c |
| Soy protein extract | 792.3 ± 0.28 a | 209.6 ± 10.73 a | 17.4 ± 1.55 c,d |
| Flaxseeds | 634.0 ± 18.95 a | 73.2 ± 3.32 a | 10.4 ± 0.44 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzoni, D.; Skřivanová, E.; Rebucci, R.; Crotti, A.; Baldi, A.; Marchetti, L.; Giromini, C. Total Phenolic Content and Antioxidant Activity of In Vitro Digested Hemp-Based Products. Foods 2023, 12, 601. https://doi.org/10.3390/foods12030601
Lanzoni D, Skřivanová E, Rebucci R, Crotti A, Baldi A, Marchetti L, Giromini C. Total Phenolic Content and Antioxidant Activity of In Vitro Digested Hemp-Based Products. Foods. 2023; 12(3):601. https://doi.org/10.3390/foods12030601
Chicago/Turabian StyleLanzoni, Davide, Eva Skřivanová, Raffaella Rebucci, Antonio Crotti, Antonella Baldi, Luca Marchetti, and Carlotta Giromini. 2023. "Total Phenolic Content and Antioxidant Activity of In Vitro Digested Hemp-Based Products" Foods 12, no. 3: 601. https://doi.org/10.3390/foods12030601
APA StyleLanzoni, D., Skřivanová, E., Rebucci, R., Crotti, A., Baldi, A., Marchetti, L., & Giromini, C. (2023). Total Phenolic Content and Antioxidant Activity of In Vitro Digested Hemp-Based Products. Foods, 12(3), 601. https://doi.org/10.3390/foods12030601