Potential Application of High Hydrostatic Pressure on the Production of Hydrolyzed Proteins with Antioxidant and Antihypertensive Properties and Low Allergenicity: A Review
Abstract
:1. Introduction
2. Effect of High Hydrostatic Pressure on Proteins
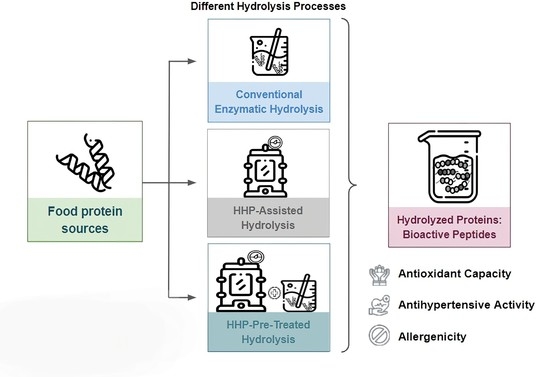
3. Utilization Strategies of HHP Associated with Enzymatic Hydrolysis
4. Effect of HHP on Proteolysis and on the Bioactivity and Allergenicity of Hydrolysates
4.1. Effect of HHP on Protein Hydrolysis
4.2. Effect of HHP on the Antihypertensive Activity of Hydrolysates
4.3. Effect of HHP on the Antioxidant Capacity of Hydrolysates
4.4. Effect of HHP on the Allergenicity of Hydrolysates
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lafarga, T.; Hayes, M. Bioactive Protein Hydrolysates in the Functional Food Ingredient Industry: Overcoming Current Challenges. Food Rev. Int. 2017, 33, 217–246. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y. Review and Perspective on Bioactive Peptides: A Roadmap for Research, Development, and Future Opportunities. J. Agric. Food Res. 2022, 9, 100353. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A Review on Health-Promoting, Biological, and Functional Aspects of Bioactive Peptides in Food Applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, J.; Malini, M.; Arun Joshy, V. A Critical Review on Food Protein Derived Antihypertensive Peptides. Drug Invent. Today 2019, 12, 474–479. [Google Scholar]
- Marciniak, A.; Suwal, S.; Naderi, N.; Pouliot, Y.; Doyen, A. Enhancing Enzymatic Hydrolysis of Food Proteins and Production of Bioactive Peptides Using High Hydrostatic Pressure Technology. Trends Food Sci. Technol. 2018, 80, 187–198. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Sadeghi Mahoonak, A.R.; Rafipour, F. Development of Animal/Plant-Based Protein Hydrolysate and Its Application in Food, Feed and Nutraceutical Industries: State of the Art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant Protein-Derived Antioxidant Peptides: Isolation, Identification, Mechanism of Action and Application in Food Systems: A Review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef] [PubMed]
- Nasri, M. Protein Hydrolysates and Biopeptides: Production, Biological Activities, and Applications in Foods and Health Benefits: A Review, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 81. [Google Scholar]
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy Protein Hydrolysates: Peptides for Health Benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef]
- Zou, Y.; Shahidi, F.; Shi, H.; Wang, J.; Huang, Y.; Xu, W.; Wang, D. Values-Added Utilization of Protein and Hydrolysates from Animal Processing by-Product Livers: A Review. Trends Food Sci. Technol. 2021, 110, 432–442. [Google Scholar] [CrossRef]
- Montesano, D.; Gallo, M.; Blasi, F.; Cossignani, L. Biopeptides from Vegetable Proteins: New Scientific Evidences. Curr. Opin. Food Sci. 2020, 31, 31–37. [Google Scholar] [CrossRef]
- Korhonen, H. Milk-Derived Bioactive Peptides: From Science to Applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Preparation, Properties, and Uses of Enzymatic Milk Protein Hydrolysates. Crit. Rev. Food Sci. Nutr. 2017, 57, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, D.; Kristoffersen, K.A.; Wubshet, S.G.; Hunnes, L.M.G.; Dalsnes, M.; Dankel, K.R.; Høst, V.; Afseth, N.K. Exploring Effects of Protease Choice and Protease Combinations in Enzymatic Protein Hydrolysis of Poultry By-Products. Molecules 2021, 26, 5280. [Google Scholar] [CrossRef]
- Cheison, S.C.; Kulozik, U. Impact of the Environmental Conditions and Substrate Pre-Treatment on Whey Protein Hydrolysis: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 418–453. [Google Scholar] [CrossRef] [PubMed]
- Abadía-García, L.; Castaño-tostado, E.; Ozimek, L.; Romero-gómez, S.; Ozuna, C.; Amaya-llano, S.L. Impact of Ultrasound Pretreatment on Whey Protein Hydrolysis by Vegetable Proteases. Innov. Food Sci. Emerg. Technol. 2016, 37, 84–90. [Google Scholar] [CrossRef]
- Tavares, T.G.; Amorim, M.; Gomes, D.; Pintado, M.E.; Pereira, C.D.; Malcata, F.X. Manufacture of Bioactive Peptide-Rich Concentrates from Whey: Characterization of Pilot Process. J. Food Eng. 2012, 110, 547–552. [Google Scholar] [CrossRef]
- Pottier, L.; Villamonte, G.; de Lamballerie, M. Applications of High Pressure for Healthier Foods. Curr. Opin. Food Sci. 2017, 16, 21–27. [Google Scholar] [CrossRef]
- Yamamoto, K. Food Processing by High Hydrostatic Pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jäger, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of High Hydrostatic Pressure Food Processing: Perspectives on Technology and Food Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266. [Google Scholar] [CrossRef]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. High Pressure Effects on Protein Structure and Function. Proteins Struct. Funct. Genet. 1996, 24, 81–91. [Google Scholar] [CrossRef]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High Pressure Enhancement of Enzymes: A Review. Enzyme Microb. Technol. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese Whey: A Potential Resource to Transform into Bioprotein, Functional/ Nutritional Proteins and Bioactive Peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Pérez-Rodríguez, L.; Pablos-Tanarro, A.; López-Fandiño, R.; Molina, E. Pepsin Treatment of Whey Proteins under High Pressure Produces Hypoallergenic Hydrolysates. Innov. Food Sci. Emerg. Technol. 2017, 43, 154–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Bi, Y.; Wang, Q.; Cheng, K.W.; Chen, F. Review: Seafood Allergy and Potential Application of High Hydrostatic Pressure to Reduce Seafood Allergenicity. Int. J. Food Eng. 2019, 15, 20180392. [Google Scholar] [CrossRef]
- Hendrickx, M.; Ludikhuyze, L.; van den Broeck, I.; Weemaes, C. Efects of High Pressure on Enzymes Related to Food Quality. Trends Food Sci. Technol. 1998, 9, 197–203. [Google Scholar] [CrossRef]
- Lullien-Pellerin, V.; Balny, C. High-Pressure as a Tool to Study Some Proteins’ Properties: Conformational Modification, Activity and Oligometric Dissociation. Innov. Food Sci. Emerg. Technol. 2002, 3, 209–221. [Google Scholar] [CrossRef]
- Meng, X.; Bai, Y.; Gao, J.; Li, X.; Chen, H. Effects of High Hydrostatic Pressure on the Structure and Potential Allergenicity of the Major Allergen Bovine B-Lactoglobulin. Food Chem. 2017, 219, 290–296. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Sihag, M.; Kaushik, R. High Pressure Processing and Its Impact on Milk Proteins: A Review. J. Dairy Sci. Technol. 2013, 2, 2319–3409. [Google Scholar]
- Yang, J.; Powers, J.R. Effects of High Pressure on Food Proteins. In High Pressure Processing of Food; Springer: New York, NY, USA, 2016; pp. 353–389. ISBN 9781493932344. [Google Scholar]
- Landim, A.P.M.; Chávez, D.W.H.; da Rosa, J.S.; Mellinger-Silva, C.; Rosenthal, A. Effect of High Hydrostatic Pressure on the Antioxidant Capacity and Peptic Hydrolysis of Whey Proteins. Ciênc. Rural. 2021, 51, e20200560. [Google Scholar] [CrossRef]
- Carullo, D.; Barbosa-Cánovas, G.V.; Ferrari, G. Changes of Structural and Techno-Functional Properties of High Hydrostatic Pressure (HHP) Treated Whey Protein Isolate over Refrigerated Storage. LWT Food Sci. Technol. 2020, 137, 110436. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Zieliński, H.; Wiczkowski, W.; Zielińska, D.; Martínez-Villaluenga, C. High-Pressure-Assisted Enzymatic Release of Peptides and Phenolics Increases Angiotensin Converting Enzyme i Inhibitory and Antioxidant Activities of Pinto Bean Hydrolysates. J. Agric. Food Chem. 2016, 64, 1730–1740. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.W.; Tang, C.H.; Wen, Q.B.; Yang, X.Q.; Li, L. Functional Properties and in Vitro Trypsin Digestibility of Red Kidney Bean (Phaseolus vulgaris L.) Protein Isolate: Effect of High-Pressure Treatment. Food Chem. 2008, 110, 938–945. [Google Scholar] [CrossRef]
- Al-Ruwaih, N.; Ahmed, J.; Mulla, M.F.; Arfat, Y.A. High-Pressure Assisted Enzymatic Proteolysis of Kidney Beans Protein Isolates and Characterization of Hydrolysates by Functional, Structural, Rheological and Antioxidant Properties. Food Sci. Technol. 2019, 100, 231–236. [Google Scholar] [CrossRef]
- Boukil, A.; Suwal, S.; Chamberland, J.; Pouliot, Y.; Doyen, A. Ultrafiltration Performance and Recovery of Bioactive Peptides after Fractionation of Tryptic Hydrolysate Generated from Pressure-Treated Β-Lactoglobulin. J. Memb. Sci. 2018, 556, 42–53. [Google Scholar] [CrossRef]
- Girgih, A.T.; Chao, D.; Lin, L.; He, R.; Jung, S.; Aluko, R.E. Enzymatic Protein Hydrolysates from High Pressure-Pretreated Isolated Pea Proteins Have Better Antioxidant Properties than Similar Hydrolysates Produced from Heat Pretreatment. Food Chem. 2015, 188, 510–516. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Alonso, E.; López-Fandiño, R. Food Hydrocolloids Antibody Binding and Functional Properties of Whey Protein Hydrolysates Obtained under High Pressure. Food Hydrocoll. 2009, 23, 593–599. [Google Scholar] [CrossRef]
- Belloque, J.; Chicón, R.; López-Fendiño, R. Unfolding and Refolding of B-Lactoglobulin Subjected to High Hydrostatic Pressure at Different PH Values and Temperatures and Its Influence on Proteolysis. J. Agric. Food Chem. 2007, 55, 5282–5288. [Google Scholar] [CrossRef]
- Ambrosi, V.; Polenta, G.; Gonzalez, C.; Ferrari, G.; Maresca, P. High Hydrostatic Pressure Assisted Enzymatic Hydrolysis of Whey Proteins. Innov. Food Sci. Emerg. Technol. 2016, 38, 294–301. [Google Scholar] [CrossRef]
- Blayo, C.; Vidcoq, O.; Lazennec, F.; Dumay, E. Effects of High Pressure Processing (Hydrostatic High Pressure and Ultra-High Pressure Homogenisation) on Whey Protein Native State and Susceptibility to Tryptic Hydrolysis at Atmospheric Pressure. Food Res. Int. 2016, 79, 40–53. [Google Scholar] [CrossRef]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. Exploiting the Effects of High Hydrostatic Pressure in Biotechnological Applications. Trends Biotechnol. 1994, 12, 493–501. [Google Scholar] [CrossRef]
- Landim, A.P.M. Aplicação de Alta Pressão Hidrostática Para Melhoria Do Processo de Hidrólise Das Proteínas Do Soro de Leite Utilizando Diferentes Proteases; Tese, Universidade Federal Rural do Rio de Janeiro: Seropedica, Brazil, 2021. [Google Scholar]
- Rutherfurd, S.M. Methodology for Determining Degree of Hydrolysis of Proteins in Hydrolysates: A Review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Mulla, M.; Al-Ruwaih, N.; Arfat, Y.A. Effect of High-pressure Treatment Prior to Enzymatic Hydrolysis on Rheological, Thermal, and Antioxidant Properties of Lentil Protein Isolate. Legume Sci. 2019, 1, e10. [Google Scholar] [CrossRef]
- Perreault, V.; Hénaux, L.; Bazinet, L.; Doyen, A. Pretreatment of Flaxseed Protein Isolate by High Hydrostatic Pressure: Impacts on Protein Structure, Enzymatic Hydrolysis and Final Hydrolysate Antioxidant Capacities. Food Chem. 2017, 221, 1805–1812. [Google Scholar] [CrossRef]
- Zeece, M.; Huppertz, T.; Kelly, A. Effect of High-Pressure Treatment on in-Vitro Digestibility of β-Lactoglobulin. Innov. Food Sci. Emerg. Technol. 2008, 9, 62–69. [Google Scholar] [CrossRef]
- Zhao, R.J.; Huo, C.Y.; Qian, Y.; Ren, D.F.; Lu, J. Ultra-High-Pressure Processing Improves Proteolysis and Release of Bioactive Peptides with Activation Activities on Alcohol Metabolic Enzymes in Vitro from Mushroom Foot Protein. Food Chem. 2017, 231, 25–32. [Google Scholar] [CrossRef]
- Singh, A.; Ramaswamy, H.S. Effect of High-Pressure Treatment on Trypsin Hydrolysis and Antioxidant Activity of Egg White Proteins. Int. J. Food Sci. Technol. 2014, 49, 269–279. [Google Scholar] [CrossRef]
- Hoppe, A.; Jung, S.; Patnaik, A.; Zeece, M.G. Effect of High Pressure Treatment on Egg White Protein Digestibility and Peptide Products. Innov. Food Sci. Emerg. Technol. 2013, 17, 54–62. [Google Scholar] [CrossRef]
- Franck, M.; Perreault, V.; Suwal, S.; Marciniak, A.; Bazinet, L.; Doyen, A. High Hydrostatic Pressure-Assisted Enzymatic Hydrolysis Improved Protein Digestion of Flaxseed Protein Isolate and Generation of Peptides with Antioxidant Activity. Food Res. Int. 2019, 115, 467–473. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, T.H. Identification and Characterization of Antioxidant Peptides from Sweet Potato Protein Hydrolysates by Alcalase under High Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2017, 43, 92–101. [Google Scholar] [CrossRef]
- Guan, H.; Diao, X.; Jiang, F.; Han, J.; Kong, B. The Enzymatic Hydrolysis of Soy Protein Isolate by Corolase PP under High Hydrostatic Pressure and Its Effect on Bioactivity and Characteristics of Hydrolysates. Food Chem. 2018, 245, 89–96. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Gomez, R.; Martinez-Villaluenga, C. High-Pressure Improves Enzymatic Proteolysis and the Release of Peptides with Angiotensin I Converting Enzyme Inhibitory and Antioxidant Activities from Lentil Proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Mu, T.; Sun, H.; Zhang, M. Digestibility and Structural Properties of Thermal and High Hydrostatic Pressure Treated Sweet Potato (Ipomoea batatas L.) Protein. Plant Foods Hum. Nutr. 2014, 69, 270–275. [Google Scholar] [CrossRef]
- Nazir, M.A.; Mu, T.H.; Zhang, M. Preparation and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Sweet Potato Protein by Enzymatic Hydrolysis under High Hydrostatic Pressure. Int. J. Food Sci. Technol. 2020, 55, 482–489. [Google Scholar] [CrossRef]
- Gomes, C.; Ferreira, D.; Carvalho, J.P.F.; Barreto, C.A.V.; Fernandes, J.; Gouveia, M.; Ribeiro, F.; Duque, A.S.; Vieira, S.I. Current Genetic Engineering Strategies for the Production of Antihypertensive ACEI Peptides. Biotechnol. Bioeng. 2020, 117, 2610–2628. [Google Scholar] [CrossRef]
- Morales-Camacho, J.I.; Espinosa-Hernández, E.; Rosas-Cárdenas, F.F.; Semería-Maitret, T.; Luna-Suárez, S. Insertions of Antihypertensive Peptides and Their Applications in Pharmacy and Functional Foods. Appl. Microbiol. Biotechnol. 2019, 103, 2493–2505. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, C.; Ye, J.; Tao, R.; Chen, H.; Cao, F. Effects of Enzymatic Hydrolysis Assisted by High Hydrostatic Pressure Processing on the Hydrolysis and Allergenicity of Proteins from Ginkgo Seeds. Food Bioproc. Technol. 2016, 9, 839–848. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Mohan, A. Mechanisms of Food Protein-Derived Antihypertensive Peptides Other than ACE Inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. Molecular Targets of Antihypertensive Peptides: Understanding the Mechanisms of Action Based on the Pathophysiology of Hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar] [CrossRef]
- Kobori, H.; Nangaku, M.; Navar, L.G.; Nishiyama, A. Intratubular Renin-Angiotensin System: From Physiology to the Pahobiology of Hypertension and Kidney Disease. Pharmacol. Rev. 2007, 59, 251–287. [Google Scholar] [CrossRef]
- Norris, R.; FitzGerald, R.J. Antihypertensive Peptides from Food Proteins: A Review. In Bioactive Food Peptides in Health and Disease Released; IntechOpen: London, UK, 2013; Volume 3, pp. 350–361. [Google Scholar]
- Mora, L.; Gallego, M.; Toldrá, F. ACEI-Inhibitory Peptides Naturally Generated in Meat and Meat Products and Their Health Relevance. Nutrients 2018, 10, 1259. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Health Relevance of Antihypertensive Peptides in Foods. Curr. Opin. Food Sci. 2018, 19, 8–14. [Google Scholar] [CrossRef]
- Chao, D.; He, R.; Jung, S.; Aluko, R.E. Effect of Pressure or Temperature Pretreatment of Isolated Pea Protein on Properties of the Enzymatic Hydrolysates. Food Res. Int. 2013, 54, 1528–1534. [Google Scholar] [CrossRef]
- Quirós, A.; Chichón, R.; Recio, I.; López-Fendiño, R. The Use of High Hydrostatic Pressure to Promote the Proteolysis and Release of Bioactive Peptides from Ovalbumin. Food Chem. 2007, 104, 1734–1739. [Google Scholar] [CrossRef]
- Aluko, R.E. Structure and Function of Plant Protein-Derived Antihypertensive Peptides. Curr. Opin. Food Sci. 2015, 4, 44–50. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Yoo, H.; Bamdad, F.; Gujral, N.; Suh, J.-W.; Sunwoo, H. High Hydrostatic Pressure-Assisted Enzymatic Treatment Improves Antioxidant and Anti-Inflammatory Properties of Phosvitin. Curr. Pharm. Biotechnol. 2017, 18, 158–167. [Google Scholar] [CrossRef]
- Piccolomini, A.F.; Iskandar, M.M.; Lands, L.C.; Kubow, S. High Hydrostatic Pressure Pre-Treatment of They Proteins Enhances Whey Protein Hydrolysate Inhibition of Oxidative Stress and IL-8 Secretion in Intestinal Epithelial Cells. Food Nutr. Res. 2012, 56, 63–69. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, B.; Miao, M.; Mu, W.; Li, Y. Combined Effects of High-Pressure and Enzymatic Treatments on the Hydrolysis of Chickpea Protein Isolates and Antioxidant Activity of the Hydrolysates. Food Chem. 2012, 135, 904–912. [Google Scholar] [CrossRef]
- Iskandar, M.M.; Landis, L.C.; Sabally, K.; Azadi, B.; Meehan, B.; Mawji, N.; Skinner, C.D.; Kubow, S. High Hydrostatic Pressure Pretreatment of Whey Protein Isolates Improves Their Digestibility and Antioxidant Capacity. Foods 2015, 4, 184–207. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited Review: Whey Proteins as Antioxidants and Promoters of Cellular Antioxidant Pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef]
- Vilela, R.M.; Lands, L.C.; Chan, H.M.; Azadi, B.; Kubow, S. High Hydrostatic Pressure Enhances Whey Protein Digestibility to Generate Whey Peptides That Improve Glutathione Status in CFTR-Deficient Lung Epithelial Cells. Mol. Nutr. Food Res. 2006, 50, 1013–1029. [Google Scholar] [CrossRef] [PubMed]
- Power-Grant, O.; McCormack, W.G.; Ramia De Cap, M.; Amigo-Benavent, M.; Fitzgerald, R.J.; Jakeman, P. Evaluation of the Antioxidant Capacity of a Milk Protein Matrix in Vitro and in Vivo in Women Aged 50-70 Years. Int. J. Food Sci. Nutr. 2016, 67, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Somkuti, J.; Smeller, L. High Pressure Effects on Allergen Food Proteins. Biophys. Chem. 2013, 183, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chizoba Ekezie, F.G.; Cheng, J.H.; Sun, D.W. Effects of Nonthermal Food Processing Technologies on Food Allergens: A Review of Recent Research Advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Bovine Milk Allergens: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Reduction of Milk Protein Antigenicity by Enzymatic Hydrolysis and Fermentation. A Review. Food Rev. Int. 2019, 37, 276–295. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of Processing on Conformational Changes of Food Proteins Related to Allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Kasera, R.; Singh, A.B.; Lavasa, S.; Prasad, K.N.; Arora, N. Enzymatic Hydrolysis: A Method in Alleviating Legume Allergenicity. Food Chem. Toxicol. 2015, 76, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, P.; Brode, V.; Sevenich, R.; Ueberham, E.; Schweiggert-Weisz, U.; Lehmann, J.; Rauh, C.; Knorr, D.; Eisner, P. High Pressure Processing Assisted Enzymatic Hydrolysis—An Innovative Approach for the Reduction of Soy Immunoreactivity. Innov. Food Sci. Emerg. Technol. 2017, 40, 58–67. [Google Scholar] [CrossRef]
- Landim, A.P.M.; Matsubara, N.K.; da Silva-Santos, J.E.; Mellinger-Silva, C.; Rosenthal, A. Application of Preliminary High-Pressure Processing for Improving Bioactive Characteristics and Reducing Antigenicity of Whey Protein Hydrolysates. Food Sci. Technol. Int. 2021, 28, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Chicón, R.; López-Fandiño, R.; Alonso, E.; Belloque, J. Proteolytic Pattern, Antigenicity, and Serum Immunoglobulin e Binding of β-Lactoglobulin Hydrolysates Obtained by Pepsin and High-Pressure Treatments. J. Dairy Sci. 2008, 91, 928–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñas, E.; Snel, H.; Floris, R.; Préstamo, G.; Gomez, R. High Pressure Can Reduce the Antigenicity of Bovine Whey Protein Hydrolysates. Int. Dairy J. 2006, 16, 969–975. [Google Scholar] [CrossRef]
- Peñas, E.; Restani, P.; Ballabio, C.; Préstamo, G.; Fiocchi, A.; Gómez, R. Assessment of the Residual Immunoreactivity of Soybean Whey Hydrolysates Obtained by Combined Enzymatic Proteolysis and High Pressure. Eur. Food Res. Technol. 2006, 222, 286–290. [Google Scholar] [CrossRef]
- Bonomi, F.; Fiocchi, A.; Frøkiær, H.; Gaiaschi, A.; Iametti, S.; Poiesi, C.; Rasmussen, P.; Restani, P.; Rovere, P. Reduction of Immunoreactivity of Bovine β-Lactoglobulin upon Combined Physical and Proteolytic Treatment. J. Dairy Res. 2003, 70, 51–59. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Chicón, R.; Belloque, J.; Recio, I.; Alonso, E.; López-Fandiño, R. Changes in the Ovalbumin Proteolysis Profile by High Pressure and Its Effect on IgG and IgE Binding. J. Agric. Food Chem. 2008, 56, 11809–11816. [Google Scholar] [CrossRef]
- López-Expósito, I.; Chicón, R.; Belloque, J.; López-Fandiño, R.; Berin, M.C. In Vivo Methods for Testing Allergenicity Show That High Hydrostatic Pressure Hydrolysates of β-Lactoglobulin Are Immunologically Inert. J. Dairy Sci. 2012, 95, 541–548. [Google Scholar] [CrossRef]
- Bøgh, K.L.; Barkholt, V.; Madsen, C.B. Characterization of the Immunogenicity and Allergenicity of Two Cow’s Milk Hydrolysates—A Study in Brown Norway Rats. Scand. J. Immunol. 2015, 81, 274–283. [Google Scholar] [CrossRef]



| Source | HHP Parameter (Pressure/Time) | Enzyme | Main Results | Reference |
|---|---|---|---|---|
| Sweet potato | 100, 200, and 300 MPa/60 min (AH) | Papain, Pepsin, and Alcalase | Pressure increased the ACE-inhibitory activity in all treatments. However, for the papain and alcalase hydrolysates, the maximum inhibitory activity was obtained with pressures lower than 200 MPa and below 300 MPa for pepsin. | [57] |
| Soybean | 80, 100, 120, 200, and 300 MPa/1, 2, 3, 4, and 5 h (AH) | Colorase PP® | The hydrolysates obtained under high pressure showed a higher ACE-inhibitory activity than conventional hydrolysis. The treatment at 200 MPa for 5 h resulted in the greatest inhibition. | [54] |
| Common bean | 100 and 200 MPa/15 min (AH) | Alcalase and Savinase | HHP-assisted hydrolysis increased the ACE-inhibitory activity at 100 and 200 MPa for alcalase and at 200 MPa for savinase, in addition to reducing the hydrolysis time. | [34] |
| Lentil | 100, 200, 300, and 400 and 500 MPa/15 min (AH) | Alcalase, Savinase, Promatex, and Colorase PP® | The treatment had no effect on the hydrolysate obtained from alcalase. The pressure of 200 MPa for promatex and 300 MPa for colorase and savinase resulted in hydrolysates with a higher ACE-inhibitory activity. | [55] |
| Peas | 200, 400, and 600 MPa/5 min (PT) | Alcalase | Pre-treatment at 600 MPa favored an increase in peptides with ACE-inhibitory capacity, even when using low enzyme concentrations. | [67] |
| Eggs | 100–400 MPa/5, 10 and 20 min (AH) 100–400 MPa/20 min (PT) | Pepsin, Chymotrypsin, and Trypsin | High pressure hydrolysis accelerated the process and increased the release of peptides identified with ACE-inhibitory activity. | [68] |
| Source | HHP Parameter (Pressure/Time) | Assay | Enzyme | Main Results | Reference |
|---|---|---|---|---|---|
| Whey | 100, 250, 400 MPa/5, 20, and 35 min (AH and PT) | ORAC and ABTS | Pepsin | PT resulted in hydrolysates with a higher antioxidant capacity than conventional treatment. | [32] |
| Lentil | 300, 400, and 600 MPa/15 min (PT) | DPPH | Alcalase | PT at 300 MPa/15 min increased the hydrolysate’s ability to reduce the DPPH radical. | [46] |
| Common bean | 300, 400, and 600 MPa/15 min (PT) | DPPH | Alcalase | PT at 300 MPa produced hydrolysates with a greater antioxidant capacity. | [36] |
| Soybean | 80, 100, 120, 200, and 300 MPa/1, 2, 3, 4, and 5 h | ABTS Reducing power | Colorase PP® | The treatment with 200 MPa/5 h resulted in a hydrolysate capable of reducing the ABTS radical by 62%. | [54] |
| Flaxseed | 100 and 300 MPa/5 and 10 min (AH) | ORAC | Trypsin | Pressure and time were important factors in increasing bioactivity, with treatment at 300 MPa/10 min increasing the antioxidant capacity by 20%. | [52] |
| Phosvitin | 50 and 100 MPa/6, 12, and 24 h (AH) | DPPH, FRAP, SRSA, and MCA | Alcalase, trypsin | HHP improved the ability to reduce the DPPH radical, the superoxide radical scavenging, and the iron reduction capacity of the alcalase hydrolysate. The iron chelation capacity was improved for alcalase and trypsin. | [71] |
| β-lactoglobulin | 100, 200, 300, and 400 and 500 MPa/15 min (AH) | ORAC | Alcalase, Savinase, Promatex, and colorase | HHP improved the antioxidant capacity of the hydrolysates. | [72] |
| Flaxseed | 600 MPa/5, 10, and 20 min (PT) | ORAC | Trypsin and trypsin-pronase | PT at 600 MPa/20 min resulted in the greatest increase in the antioxidant activity of the hydrolysates. | [47] |
| Sweet potato | 100, 200, and 300 MPa/ 30 and 60 min (AH) | ORAC | Alcalase | The hydrolysate obtained in the HHP-assisted hydrolysis using 300 MPa/20 min resulted in the highest antioxidant capacity. | [7] |
| Common bean | 100 and 200 MPa/15 min (AH) | ORAC, FRAP, ABTS | Alcalase and Savinase | The highest ORAC and ABTS values of the hydrolysates were observed in the treatments at 200 MPa for savinase and 100 MPa for alcalase. HHP had no influence on the ability of the hydrolysates to reduce iron for both enzymes. | [34] |
| Lentil | 100, 200, 300, 400, and 500 MPa/15 min (AH) | ORAC | Alcalase, Savinase, Promatex. Colorase | Treatment at 100 MPa produced hydrolysates with a higher antioxidant capacity from alcalase, and 300 MPa exhibited greater effects for savinase and colorase. | [55] |
| Peas | 200, 400, 600 MPa/5 min (PT) | ORAC, DPPH, FRAP, MCA, SRSA | Alcalase | Improved DPPH scavenging capacity at 400 MPa. Improved ORAC activity at 400 and 600 MPa. | [38] |
| Chickpea | 100, 200, 300, 400, 500, and 600 MPa (PT); 100, 200, 300 MPa (AH) | SRSA, FRAP | Alcalase | PT 300 and 400 MPa and assisted hydrolysis at 200 MPa/30 min were more effective in increasing the antioxidant capacity of hydrolysates. | [73] |
| Source | HHP Parameter (Pressure/Time) | Enzyme | Main Results | Reference |
|---|---|---|---|---|
| Whey | 100, 250, 400 MPa/5, 20, and 35 min (PT) | Novo Pro-D and Ficin | PT contributes to reducing the antigenicity of the hydrolysates obtained by ficin and reduced the hydrolysis time from 60 min to 15 min necessary to achieve a complete reduction in immunoreactivity. | [85] |
| Whey | 400 MPa/30 min (AH) | Pepsin | Maximum hydrolysis with peptides ranging from 10 to 3 kDa (50%), with a reduction in intact allergens and no induction of clinical signs in sensitized mice. | [25] |
| β-lactoglobulin | 100, 200, 300, and 400 MPa/120 min (PT and AH) | Pepsin | PT did not influence the immunoreactivity of hydrolysates. Assisted hydrolysis progressively reduced antigenicity with increasing incubation times and pressures. | [86] |
| Whey | 200 and 400 MPa/10, 30, and 60 min (AH) | Pepsin and Chymotrypsin | HHP favored a reduction in b-LG immunoreactivity with the use of the pepsin enzyme, which was progressive with the incubation time and increasing pressure (400 MPa/30 min). | [39] |
| Whey | 100 and 200 MPa/min (PT and AH) | Alcalase, Neutrase, Colorase 7089, and Colocarase PN-L | The treatment influenced the reduction in antigenicity only in the hydrolysate obtained from the enzyme Colorase PN-L at 300 MPa. | [87] |
| Soybean | 100,200, and 300 MPa/15 min (AH) | Alcalase, Neutrase, and Colorase | The treatment using the pressure of 300 MPa contributed to reducing the immunoreactivity of the hydrolysates obtained from Colorase. | [88] |
| β-lactoglobulin | 600 MPa/10 min (PT) | Trypsin and Chymotrypsin | Decreased immunoreactivity after combination treatment, which was greater when chymotrypsin was used. | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landim, A.P.M.; Tiburski, J.H.; Mellinger, C.G.; Juliano, P.; Rosenthal, A. Potential Application of High Hydrostatic Pressure on the Production of Hydrolyzed Proteins with Antioxidant and Antihypertensive Properties and Low Allergenicity: A Review. Foods 2023, 12, 630. https://doi.org/10.3390/foods12030630
Landim APM, Tiburski JH, Mellinger CG, Juliano P, Rosenthal A. Potential Application of High Hydrostatic Pressure on the Production of Hydrolyzed Proteins with Antioxidant and Antihypertensive Properties and Low Allergenicity: A Review. Foods. 2023; 12(3):630. https://doi.org/10.3390/foods12030630
Chicago/Turabian StyleLandim, Ana Paula Miguel, Julia Hauck Tiburski, Caroline Grassi Mellinger, Pablo Juliano, and Amauri Rosenthal. 2023. "Potential Application of High Hydrostatic Pressure on the Production of Hydrolyzed Proteins with Antioxidant and Antihypertensive Properties and Low Allergenicity: A Review" Foods 12, no. 3: 630. https://doi.org/10.3390/foods12030630
APA StyleLandim, A. P. M., Tiburski, J. H., Mellinger, C. G., Juliano, P., & Rosenthal, A. (2023). Potential Application of High Hydrostatic Pressure on the Production of Hydrolyzed Proteins with Antioxidant and Antihypertensive Properties and Low Allergenicity: A Review. Foods, 12(3), 630. https://doi.org/10.3390/foods12030630