Characterizations and the Mechanism Underlying Cryoprotective Activity of Peptides from Enzymatic Hydrolysates of Pseudosciaena crocea
Abstract
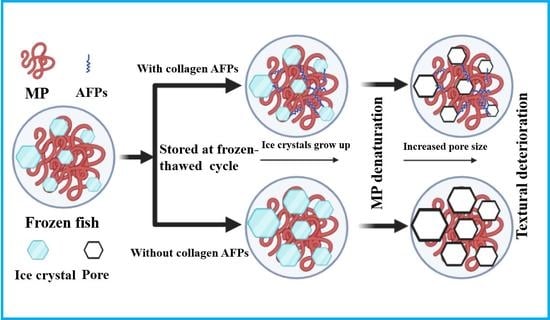
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Polypeptide from P. crocea
2.3. Determination of Protein Concentration
2.4. Sample Pre-Treatment
2.5. Screening of Antifreeze Peptides
2.5.1. Molecular Weight Distribution (MWD) and Amino Acid Content Determination
2.5.2. Measurement of In Vitro Antioxidant Activity
2.5.3. Texture Properties Analysis
2.6. Determination of Physical Properties
2.6.1. Water-Holding Capacity
2.6.2. Colour
2.6.3. Electronic Nose
2.6.4. Electronic Tone
2.7. Extraction of Turbot Myofibrillar Protein
2.8. Determination of Myofibrillar Protein Conformation
2.8.1. Fluorescence Spectroscopy
2.8.2. UV Absorption Spectroscopy
2.8.3. Circular Dichroism
2.9. Determination of MFP Aggregation
2.9.1. Determination of Surface Hydrophobicity of MFP
2.9.2. Particle Size and Zeta Potential
2.10. Determination of the Degree of Myofibrillar Protein Oxidation
2.10.1. Protein Solubility
2.10.2. Total Sulfhydryl (T-SH) Content
2.10.3. Ca2+-ATPase Activity
2.10.4. Carbonyl Content
2.10.5. Dityrosine Content
2.11. Statistical Analysis
3. Results and Discussion
3.1. Screening of Antifreeze Peptides
3.1.1. The Composition of MWD and Amino Acids
3.1.2. In Vitro Antioxidant Properties
3.1.3. TPA
3.2. Determination of Physical Properties
3.3. Circular Dichroism
3.4. Fluorescence Spectroscopy
3.5. UV Absorption Spectroscopy
3.6. Determination of Surface Hydrophobicity of MFP
3.7. Zeta Potential and Particle Size
3.8. Carbonyl Content
3.9. Dityrosine Content
3.10. T-SH Content
3.11. Protein Solubility
3.12. Ca2+-ATPase Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Liu, Q.; Han, Y.N. Assessing the Fishery Resource Status of China’s Coastal Waters Using Surplus Production Models. J. Ocean. Univ. China 2021, 20, 1236–1244. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, X.C. Effect of heat extraction on water-soluble taste substances in processing products of chilled large yellow croaker (Pseudosciaena crocea). Food Sci. Nutr. 2019, 7, 3863–3872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.J.; Cai, J.J.; Cai, L.; Qu, H.D.; Yang, M.; Zhang, M. Cloning and expression of a hepcidin gene from a marine fish (Pseudosciaena crocea) and the antimicrobial activity of its synthetic peptide. Peptides 2009, 30, 638–646. [Google Scholar] [CrossRef]
- Farvin, K.; Grejsen, H.D.; Jacobsen, C. Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): Effect on lipid and protein oxidation. Food Chem. 2012, 131, 843–851. [Google Scholar] [CrossRef]
- Hernández, M.D.; López, M.B.; Alvarez, A.; Ferrandini, E.; García, B.G.; Garrido, M.D. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 2009, 114, 237–245. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, Z.; Sun, D.-W. Naturally sourced biosubstances for regulating freezing points in food researches: Fundamentals, current applications and future trends. Trends Food Sci. Technol. 2020, 95, 131–140. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y. Prevention of protein oxidation and enhancement of gel properties of silver carp (Hypophthalmichthys molitrix) surimi by addition of protein hydrolysates derived from surimi processing by-products. Food Chem. 2020, 316, 126343. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Herrero, M.M.; Duflos, G.; Malle, P.; Bouquelet, S. Collagenase activity and protein hydrolysis as related to spoilage of iced cod (Gadus morhua). Food Res. Int. 2003, 36, 141–147. [Google Scholar] [CrossRef]
- Donald, G.A.M.; Lanier, T.C. Actomyosin Stabilization to Freeze-Thaw and Heat Denaturation by Lactate Salts. J. Food Sci. 1994, 59, 101–105. [Google Scholar] [CrossRef]
- Mochizuki, K.; Molinero, V. Antifreeze Glycoproteins Bind Reversibly to Ice via Hydrophobic Groups. J. Am. Chem. Soc. 2018, 140, 4803–4811. [Google Scholar] [CrossRef]
- Ustun, N.S.; Turhan, S. Antifreeze Proteins: Characteristics, Function, Mechanism of Action, Sources and Application to Foods. J. Food Process. Preserv. 2015, 39, 3189–3197. [Google Scholar] [CrossRef]
- Bianco, V.; Espinosa, J.R.; Vega, C. Antifreeze proteins and homogeneous nucleation: On the physical determinants impeding ice crystal growth. J. Chem. Phys. 2020, 153, 91–102. [Google Scholar] [CrossRef]
- Fu, W.; Wang, P.; Chen, Y.; Lin, J.; Zheng, B.; Zeng, H.; Zhang, Y. Preparation, primary structure and antifreeze activity of antifreeze peptides from Scomberomorus niphonius skin. LWT-Food Sci. Technol. 2019, 101, 670–677. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Chen, L.; Zhou, Y.; Wu, J. Preparation, isolation and hypothermia protection activity of antifreeze peptides from shark skin collagen. LWT-Food Sci. Technol. 2014, 55, 210–217. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, H.; Wang, Z.; Fan, F.; Shi, P.; Tu, M.; Du, M. Isolation and characterization of peptides from mytilus edulis with osteogenic activity in mouse MC3T3-E1 preosteoblast cells. J. Agric. Food Chem. 2019, 67, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Yang, W.G.; Wei, H.M.; Deng, S.G.; Yu, X.X.; Huang, T. The mechanisms and applications of cryoprotectants in aquatic products: An overview. Food Chem. 2023, 408, 135202. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M. Determination of Serum Proteins by Means of the Biuret Reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Tang, N.; Dong, S.T.; Sun, B.; Liu, J.B. Optimisation of antioxidant peptide preparation from corn gluten meal. J. Sci. Food Agric. 2013, 93, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Bhat, F.M.; Riar, C.S. Studies on effect of temperature and time on textural and rheological properties of starch isolated from traditional rice cultivars of Kashmir (India). J. Texture Stud. 2017, 48, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Cao, M.; Cao, A.; Wang, J.; Cai, L.; Regenstein, J.; Ruan, Y.; Li, X. Effect of magnetic nanoparticles plus microwave or far-infrared thawing on protein conformation changes and moisture migration of red seabream (Pagrus Major) fillets. Food Chem. 2018, 266, 498–507. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, W.; Jiang, Q. Aggregation and structural changes of silver carp actomyosin as affected by mild acidification with d-gluconic acid δ-lactone. Food Chem. 2012, 134, 1005–1010. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Q.; Hu, T.; Xiong, S.; Zhao, S. Effects and mechanism of modified starches on the gel properties of myofibrillar protein from grass carp. Int. J. Biol. Macromol. 2014, 64, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Li, Q.; Luo, Y. Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp (Aristichthys nobilis) affected by frozen storage and a hydroxyl radical-generation oxidizing system. Food Chem. 2017, 223, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Beliciu, C.M.; Moraru, C.I. The effect of protein concentration and heat treatment temperature on micellar casein–soy protein mixtures. Food Hydrocoll. 2011, 25, 1448–1460. [Google Scholar] [CrossRef]
- Davies, K.; Delsignore, M.E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J. Biol. Chem. 1987, 262, 9908–9913. [Google Scholar] [CrossRef]
- Wubshe, S.G.; Måge, I.; Böcker, U.; Lindberg, D.; Knutsen, S.H.; Riede, A.; Rodrigueza, D.A.; Afseth, N.K. FTIR as a rapid tool for monitoring molecular weight distribution during enzymatic protein hydrolysis of food processing by-products. Anal. Methods 2017, 9, 4247–4254. [Google Scholar] [CrossRef]
- Damodaran, S. Inhibition of ice crystal growth in ice cream mix by gelatin hydrolysate. J. Agric. Food Chem. 2007, 55, 10918–10923. [Google Scholar] [CrossRef]
- Wanga, W.; Chen, M.; Wu, J.; Wang, S. Hypothermia protection effect of antifreeze peptides from pigskin collagen on freeze-dried Streptococcus thermophiles and its possible action mechanism. LWT-Food Sci. Technol. 2015, 63, 878–885. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Wang, S.; Wang, Z.; Wu, Y.; Guo, X. Laboratory-scale extraction and characterization of ice-binding sericin peptides. Eur. Food Res. Technol. 2013, 236, 637–646. [Google Scholar] [CrossRef]
- Wiriyaphan, C.; Chitsomboon, B.; Yongsawadigul, J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem. 2012, 132, 104–111. [Google Scholar] [CrossRef]
- Cheung, I.; Cheung, L.; Tan, N.Y.; Li-Chan, E. The role of molecular size in antioxidant activity of peptide fractions from Pacific hake (Merluccius productus) hydrolysates. Food Chem. 2012, 134, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of freezing under electrostatic field on the quality of lamb meat. Innov. Food Sci. Emerg. Technol. 2016, 37, 68–73. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Vieira, C.; Diaz, M.T.; Martinez, B.; Garcia-Cachan, M.D. Effect of frozen storage conditions (temperature and length of storage) on microbiological and sensory quality of rustic crossbred beef at different states of ageing. Meat Sci. 2009, 83, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Hamid, N.; Liu, T.; Sarojini, V. Effect of Antifreeze Peptide Pretreatment on Ice Crystal Size, Drip Loss, Texture, and Volatile Compounds of Frozen Carrots. J. Agric. Food Chem. 2016, 64, 4327. [Google Scholar] [CrossRef] [PubMed]
- Filgueras, R.S.; Gatellier, P.; Zambiazi, R.C.; Santé-Lhoutellier, V. Effect of frozen storage duration and cooking on physical and oxidative changes in M. Gastrocnemius pars interna and M. Iliofiburalis of rhea americana. Meat Sci. 2011, 88, 645–651. [Google Scholar] [CrossRef]
- Saeed, S.; Fawthrop, S.A.; Howell, N.K. Electron spin resonance (ESR) study on free radical transfer in fish lipid-protein interaction. J. Sci. Food Agric. 1999, 79, 1809–1816. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Ngadi, M.O.; Simpson, B.K.; Simpson, M.V. Pulsed Electric Field Induced Structural Modification Of Soy Protein Isolate As Studied By Fluorescence Spectroscopy. J. Food Process. Preserv. 2011, 35, 563–570. [Google Scholar] [CrossRef]
- Nian, L.; Cao, A.; Cai, L. Investigation of the antifreeze mechanism and effect on quality characteristics of largemouth bass (Micropterus salmoides) during F-T cycles by hAFP. Food Chem. 2020, 325, 126918. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, H.X.; Yang, H.C.; Xiang, X.W.; Li, H.B.; Deng, S.G. Cryoprotective roles of trehalose and alginate oligosaccharides during frozen storage of peeled shrimp (Litopenaeus vannamei). Food Chem. 2017, 228, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Ishihara, T.; Hara, K.; Osatomi, K.; Khan, M.A.; Nozaki, Y. Effect of proteolytic squid protein hydrolysate on the state of water and dehydration-induced denaturation of lizard fish myofibrillar protein. J. Agric. Food Chem. 2003, 51, 4769. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.T.; Li, D.; Augustin, M.A. Deamidated wheat protein–dextran Maillard conjugates: Effect of size and location of polysaccharide conjugated on steric stabilization of emulsions at acidic pH. Food Hydrocoll. 2011, 25, 1424–1432. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Yang, W.; Xue, C. Effects of Ozone-Induced Oxidation on the Physicochemical Properties of Myofibrillar Proteins Recovered from Bighead Carp (Hypophthalmichthys nobilis). Food Bioprocess Technol. 2015, 8, 181–190. [Google Scholar] [CrossRef]
- Jorgensen, B.M.; Nielsen, M.K. Quantitative Relationship between Trimethylamine Oxide Aldolase Activity and Formaldehyde Accumulation in White Muscle from Gadiform Fish during Frozen Storage. J. Agric. Food Chem. 2004, 53, 3814–3822. [Google Scholar]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Giustarini, D.; Portinaro, N.; Badalamenti, S.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A central role for intermolecular dityrosine cross-linking of fibrinogen in high molecular weight advanced oxidation protein product (AOPP) formation. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2015, 1850, 1–12. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [Green Version]
- Sultanbawa, Y.; Li-Chan, E.C.Y. Structural changes in natural actomyosin and surimi from ling cod (Ophiodon elongatus) during frozen storage in the absence or presence of cryoprotectants. J. Agric. Food Chem. 2001, 49, 4716–4725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Benjakul, S.; Ke, P.; Jie, G.; Xin, L. Cryoprotective effects of trehalose and sodium lactate on tilapia (Sarotherodon nilotica) surimi during frozen storage. Food Chem. 2006, 96, 96–103. [Google Scholar] [CrossRef]
- Ko, W.-C.; Shi, H.-Z.; Chang, C.-K.; Huang, Y.-H.; Chen, Y.-A.; Hsieh, C.-W. Effect of adjustable parallel high voltage on biochemical indicators and actomyosin Ca2+-ATPase from tilapia (Orechromis niloticus). LWT-Food Sci. Technol. 2016, 69, 417–423. [Google Scholar] [CrossRef]
- Lv, M.; Mei, K.; Zhang, H.; Xu, D.; Yang, W. Effects of electron beam irradiation on the biochemical properties and structure of myofibrillar protein from Tegillarca granosa meat. Food Chem. 2018, 254, 64–69. [Google Scholar] [CrossRef] [PubMed]





| Total Amino Acid | Content (%) P-H | Content (%) T-H | Content (%) N-P |
|---|---|---|---|
| Hydrophilic amino acid a | 47.26 | 51.87 | 39.14 |
| Polar amino acids without a charge b | 24.07 | 27.31 | 19.19 |
| Basic amino acids c | 18.74 | 16.46 | 11.28 |
| Acidic amino acids d | 4.46 | 8.1 | 8.67 |
| Aromatic amino acids e | 3.93 | 6.18 | 6.84 |
| Branched chain amino acids f | 2.42 | 11.31 | 13.22 |
| Essential amino acids g | 33.15 | 34.63 | 33.23 |
| Samples | Hardness | Elasticity | Cohesion | Adhesion | Chewiness | Reversibility |
|---|---|---|---|---|---|---|
| FF | 50.87 ± 9.12 a | 1 ± 0.05 a | 0.48 ± 0.02 a | 24.11 ± 3.19 a | 21.68 ± 3.64 a | 0.27 ± 0.02 a |
| Control | 35.30 ± 5.68 cd | 0.94 ± 0.04 c | 0.41 ± 0.04 cd | 15.48 ± 2.69 c | 15.18 ± 2.49 c | 0.20 ± 0.02 cd |
| PH | 35.51 ± 5.51 cd | 0.94 ± 0.03 bc | 0.41 ± 0.04 d | 15.61 ± 2.85 c | 15.60 ± 3.12 bc | 0.23 ± 0.03 b |
| TH | 44.41 ± 5.5 ab | 0.98 ± 0.04 ab | 0.46 ± 0.02 ab | 20.15 ± 1.18 ab | 19.23 ± 1.63 ab | 0.23 ± 0.02 ab |
| NP | 32.22 ± 8.08 d | 0.94 ± 0.04 bc | 0.44 ± 0.03 abc | 15.26 ± 2.34 c | 15.09 ± 3.01 c | 0.22 ± 0.02 ab |
| CP | 45.47 ± 5.62 ab | 0.96 ± 0.04 abc | 0.45 ± 0.04 ab | 18.79 ± 3.74 bc | 17.38 ± 4.26 bc | 0.19 ± 0.03 c |
| CP-TH | 41.73 ± 5.64 bc | 0.93 ± 0.04 c | 0.44 ± 0.03 bcd | 17.34 ± 4.54 bc | 16.11 ± 4.28 bc | 0.19 ± 0.02 c |
| CP-TH2 | 40.86 ± 5.65 bc | 0.93 ± 0.05 c | 0.44 ± 0.03 bcd | 16.97 ± 4.86 bc | 15.73 ± 4.47 bc | 0.20 ± 0.02 c |
| Samples | L* | a* | b* | Thawing Loss (%) | Cooking Loss (%) | Centrifugal Loss (%) |
|---|---|---|---|---|---|---|
| FF | 54.09 ± 0.05 a | 2.70 ± 0.17 a | 1.14 ± 0.11 d | -- | 5.19 ± 0.9 d | 2.05 ± 0.2 d |
| TH | 50.84 ± 0.89 b | 1.31 ± 0.04 b | 2.17 ± 0.12 c | 6.81 ± 0.42 c | 8.23 ± 0.52 c | 5.4 ± 0.55 c |
| CP | 49.82 ± 0.96 b | 1.28 ± 0.1 b | 2.21 ± 0.09 c | 6.22 ± 0.79 c | 8.74 ± 0.55 c | 5.25 ± 0.87 c |
| TH-CP | 46.16 ± 0.95 c | 1.14 ± 0.14 b | 2.76 ± 0.07 b | 9.14 ± 0.61 b | 11.11 ± 0.62 b | 6.43 ± 0.34 ab |
| Control | 43.09 ± 0.78 d | 0.77 ± 0.06 c | 3.03 ± 0.18 a | 12.55 ± 0.9 a | 13.3 ± 0.14 a | 7.21 ± 0.59 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhu, Z.; Tu, M.; Chang, J.; Han, S.; Han, L.; Chen, H.; Tan, Z.; Du, M.; Li, T. Characterizations and the Mechanism Underlying Cryoprotective Activity of Peptides from Enzymatic Hydrolysates of Pseudosciaena crocea. Foods 2023, 12, 875. https://doi.org/10.3390/foods12040875
Xu Z, Zhu Z, Tu M, Chang J, Han S, Han L, Chen H, Tan Z, Du M, Li T. Characterizations and the Mechanism Underlying Cryoprotective Activity of Peptides from Enzymatic Hydrolysates of Pseudosciaena crocea. Foods. 2023; 12(4):875. https://doi.org/10.3390/foods12040875
Chicago/Turabian StyleXu, Zhe, Zhixuan Zhu, Maolin Tu, Jiale Chang, Shiying Han, Lingyu Han, Hui Chen, Zhijian Tan, Ming Du, and Tingting Li. 2023. "Characterizations and the Mechanism Underlying Cryoprotective Activity of Peptides from Enzymatic Hydrolysates of Pseudosciaena crocea" Foods 12, no. 4: 875. https://doi.org/10.3390/foods12040875
APA StyleXu, Z., Zhu, Z., Tu, M., Chang, J., Han, S., Han, L., Chen, H., Tan, Z., Du, M., & Li, T. (2023). Characterizations and the Mechanism Underlying Cryoprotective Activity of Peptides from Enzymatic Hydrolysates of Pseudosciaena crocea. Foods, 12(4), 875. https://doi.org/10.3390/foods12040875