The Bioaccessibility of Yak Bone Collagen Hydrolysates: Focus on Analyzing the Variation Regular of Peptides and Free Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
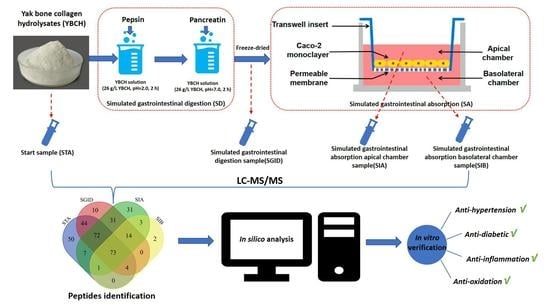
2.1. Chemical Agents and Preparation of YBCH
2.2. Simulated Gastrointestinal Digestion (SD)
2.3. Simulated Intestine Absorption (SA)
2.3.1. Cell Culture
2.3.2. Cytotoxicity Test
2.3.3. Transport Studies
2.4. Characterization of the Samples
2.5. Characterization of Bioavailable Bioactive Peptides
2.5.1. In Silico Prediction
2.5.2. In Vitro Verification
2.6. Statistical Analysis
3. Results
3.1. Molecular Weight Distribution and Concentration of Peptides
3.2. Free Amino Acids Alteration
3.3. Transport Study
3.4. Identification of Peptides
3.5. Prediction of the Biological Activity of Bioavailable Peptides
3.6. Verification of the Biological Activity of Bioavailable Peptides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, H.; Fan, H.B.; Chalamaiah, M.; Wu, J.P. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food. Chem. 2019, 301, 125222. [Google Scholar] [CrossRef]
- Lee, E.J.; Hur, J.; Ham, S.A.; Jo, Y.; Lee, S.; Choi, M.J.; Seo, H.G. Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int. J. Biol. Macromol. 2017, 104, 281–286. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Song, S.; Wang, X.; Qin, Y.; Si, S.; Guo, Y. Combined oral administration of bovine collagen peptides with calcium citrate inhibits bone loss in ovariectomized rats. PLoS ONE 2015, 10, e0135019. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; Konig, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Zhang, C.; Jia, W.; Shen, Q.; Qin, X.; Zhang, H.; Zhu, L. Metabolomics strategy reveals the osteogenic mechanism of yak (Bos grunniens) bone collagen peptides on ovariectomy-induced osteoporosis in rats. Food. Funct. 2020, 11, 1498–1512. [Google Scholar] [CrossRef]
- Gao, S.; Hong, H.; Zhang, C.; Wang, K.; Zhang, B.; Han, Q.-a.; Liu, H.; Luo, Y. Immunomodulatory effects of collagen hydrolysates from yak (Bos grunniens) bone on cyclophosphamide-induced immunosuppression in BALB/c mice. J. Funct. Food 2019, 60. [Google Scholar] [CrossRef]
- Li, F.; Jia, D.; Yao, K. Amino acid composition and functional properties of collagen polypeptide from Yak (Bos grunniens) bone. LWT.-Food. Sci. Technol. 2009, 42, 945–949. [Google Scholar] [CrossRef]
- Ye, M.; Jia, W.; Zhang, C.; Shen, Q.; Zhu, L.; Wang, L. Preparation, identification and molecular docking study of novel osteoblast proliferation-promoting peptides from yak (Bos grunniens) bones. RSC. Adv. 2019, 9, 14627–14637. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, K.; Gao, S.; Hong, H.; Zhang, L.; Liu, H.; Feng, L.; Luo, Y. Purification and characterization of antioxidant peptides from yak (Bos grunniens) bone hydrolysates and evaluation of cellular antioxidant activity. J. Food. Sci. Technol. 2021, 58, 3106–3119. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, C.; Hu, B.; Zhu, L.; Yang, Y.; Liu, F.; Gu, Z.; Xin, Y.; Zhang, L. Simulated gastrointestinal digestion of yak bone collagen hydrolysates and insights into its effects on gut microbiota composition in mice. Food. Biosci. 2021, 44, 101463. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, B.; Zhu, L.; Yang, Y.; Liu, C.; Liu, F.; Shi, Y.; Li, M.; Gu, Z.; Xin, Y.; et al. Microbiome-metabolomics insights into the feces of high-fat diet mice to reveal the anti-obesity effects of yak (Bos grunniens) bone collagen hydrolysates. Food. Res. Int. 2022, 156, 111024. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Abioye, R.O.; Okagu, I.U.; Obeme-Nmom, J.I. Bioaccessibility of bioactive peptides: Recent advances and perspectives. Curr. Opin. Food. Sci. 2021, 39, 182–189. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. Infogest static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Ningrum, A.; Wardani, D.; Vanidia, N.; Syarifudin, A.; Ekafitri, R.; Kristanti, D.; Setiaboma, W.; Siti, H.; Munawaroh, H. In Silico Approach of Glycinin and Conglycinin Chains of Soybean By-Product (Okara) Using Papain and Bromelain. Molecules 2022, 27, 6855. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Food. 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Feng, M.; Betti, M. Transepithelial transport efficiency of bovine collagen hydrolysates in a human Caco-2 cell line model. Food. Chem. 2017, 224, 242–250. [Google Scholar] [CrossRef]
- Fu, Q.X.; Wang, H.Z.; Xia, M.X.; Deng, B.; Shen, H.Y.; Ji, G.; Li, G.W.; Xie, Y. The effect of phytic acid on tight junctions in the human intestinal Caco-2 cell line and its mechanism. Eur. J. Pharm. Sci. 2015, 80, 1–8. [Google Scholar] [CrossRef]
- Trigo, J.P.; Engstrom, N.; Steinhagen, S.; Juul, L.; Harrysson, H.; Toth, G.B.; Pavia, H.; Scheers, N.; Undeland, I. In vitro digestibility and Caco-2 cell bioavailability of sea lettuce (Ulva fenestrata) proteins extracted using pH-shift processing. Food Chem. 2021, 356, 129683. [Google Scholar]
- Turner, P.C.; Wu, Q.K.; Piekkola, S.; Gratz, S.; Mykkanen, H.; El-Nezami, H. Lactobacillus rhamnosus strain GG restores alkaline phosphatase activity in differentiating Caco-2 cells dosed with the potent mycotoxin deoxynivalenol. Food. Chem. Toxicol. 2008, 46, 2118–2123. [Google Scholar] [CrossRef]
- Puchalska, P.; Concepcion Garcia, M.; Luisa Marina, M. Identification of native angiotensin-I converting enzyme inhibitory peptides in commercial soybean based infant formulas using HPLC-Q-ToF-MS. Food. Chem. 2014, 157, 62–69. [Google Scholar] [CrossRef]
- Ma, Y.; Hou, Y.; Han, B.; Xie, K.; Zhang, L.; Zhou, P. Peptidome comparison following gastrointestinal digesta of bovine versus caprine milk serum. J. Dairy. Sci. 2021, 104, 47–60. [Google Scholar] [CrossRef]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the improved discovery and design of functional peptides: Common features of diverse classes permit generalized prediction of bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef] [Green Version]
- Manayalan, B.; Basith, S.; Shin, T.H.; Wei, L.Y.; Lee, G. MAHTPred: A sequence-based meta-predictor for improving the prediction of anti-hypertensive peptides using effective feature representation. Bioinformatics 2019, 35, 2757–2765. [Google Scholar] [CrossRef]
- Charoenkwan, P.; Nantasenamat, C.; Hasan, M.M.; Moni, M.A.; Lio, P.; Manavalan, B.; Shoombuatong, W. StackDPPIV: A novel computational approach for accurate prediction of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Methods 2021, 204, 189–198. [Google Scholar] [CrossRef]
- Manavalan, B.; Shin, T.H.; Kim, M.O.; Lee, G. AIPpred: Sequence-Based Prediction of Anti-inflammatory Peptides Using Random Forest. Front. Pharmacol. 2018, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; Garcia-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhang, L.; Tao, J.; Chi, C.F.; Wang, B. Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs). Food Res. Int. 2019, 121, 197–204. [Google Scholar] [CrossRef]
- Ahn, C.B.; Je, J.Y.; Cho, Y.S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Venkatesan, K.; Nazeer, R.A. Antioxidant Activity of Purified Protein Hydrolysates from Northern Whiting Fish (Sillago sihama) Muscle. Int. J. Pept. Res. Ther. 2013, 20, 209–219. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, C.; Zhu, L.; Jia, W.; Shen, Q. Yak (Bos grunniens) bones collagen-derived peptides stimulate osteoblastic proliferation and differentiation via the activation of Wnt/β-catenin signaling pathway. J. Sci. Food. Agric. 2020, 100, 2600–2609. [Google Scholar] [CrossRef]
- Zhong, C.; Sun, L.C.; Yan, L.J.; Lin, Y.C.; Liu, G.M.; Cao, M.J. Production, optimisation and characterisation of angiotensin converting enzyme inhibitory peptides from sea cucumber (Stichopus japonicus) gonad. Food. Funct. 2018, 9, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Bhagat, D.; Mahalwal, M.; Sharma, N.; Raghava, G.P.S. AntiCP 2.0: An updated model for predicting anticancer peptides. Brief. Bioinform. 2021, 22, bbaa153. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Tanaka, M.; Koyanagi, R.; Shen, W.; Matsui, T. Structural Design of Oligopeptides for Intestinal Transport Model. J. Agric. Food. Chem. 2016, 64, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, B. Effect of molecular weight on the transepithelial transport and peptidase degradation of casein-derived peptides by using Caco-2 cell model. Food. Chem. 2017, 218, 1–8. [Google Scholar] [CrossRef]
- Xu, Q.B.; Hong, H.; Wu, J.P.; Yan, X.H. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends. Food. Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Lennernas, H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica 2007, 37, 1015–1051. [Google Scholar] [CrossRef]
- Komin, A.; Russell, L.M.; Hristova, K.A.; Searson, P.C. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: Mechanisms and challenges. Adv. Drug. Deliver. Rev. 2017, 110, 52–64. [Google Scholar] [CrossRef]
- Christina, E.L.; Michèle, M.I.; Stan, K. Assessment of bioavailability after In Vitro digestion and first pass metabolism of bioactive peptides from collagen hydrolysates. Curr. Issues. Mol. Biol. 2021, 43, 1592–1605. [Google Scholar]
- Sato, K. Structure, Content, and Bioactivity of Food-Derived Peptides in the Body. J. Agric. Food. Chem. 2018, 66, 3082–3085. [Google Scholar] [CrossRef]
- Sato, K.; Nisimura, R.; Suzuki, Y.; Motoi, H.; Nakamura, Y.; Ohtsuki, K.; Kawabata, M. Occurrence of indigestible pyroglutamyl peptides in an enzymatic hydrolysate of wheat gluten prepared on an industrial scale. J. Agric. Food. Chem. 1998, 46, 3403–3405. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.Y.; Yu, Z.P.; Ma, S.T.; Du, Z.Y.; Zhang, T.; Liu, J.B. Importance of terminal amino acid residues to the transport of oligopeptides across the caco-2 cell monolayer. J. Agric. Food. Chem. 2017, 65, 7705–7712. [Google Scholar] [CrossRef] [PubMed]





| Amino Acid Sequence | BAP_Score | Intensity (×109) | Length | Mol wt | Hydrophobicity | Hydrophilicity | Charge | pI | Collagen Type | Toxin | Allergen | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | GEAGPAGPAGPAGPR | 0.81 | 726.48 | 15 | 1261.56 | −0.06 | 0.27 | 0 | 6.00 | alpha-2 | Non | Yes |
| GGGPGPM | 0.90 | 233.89 | 7 | 571.75 | 0.11 | −0.19 | 0 | 5.52 | alpha-2 | Non | Non | |
| AGPAGPIGPV | 0.80 | 38.67 | 10 | 835.11 | 0.2 | −0.43 | 0 | 5.57 | alpha-1 | Non | Yes | |
| GPPGPAGPAG | 0.88 | 19.90 | 10 | 776.98 | 0.06 | −0.13 | 0 | 5.52 | alpha-1 | Non | Non | |
| GPPGPMGPPGLAGPPGESG | 0.89 | 19.69 | 19 | 1629.1 | 0.04 | −0.02 | −1 | 4.00 | alpha-1 | Non | Yes | |
| GPPGPMGPPG | 0.95 | 15.40 | 10 | 863.14 | 0.09 | −0.1 | 0 | 5.52 | alpha-1 | Non | Yes | |
| ADA | AGPAGPAGPAGPR | 0.88 | 126.00 | 13 | 1075.36 | −0.03 | 0.08 | 1 | 9.79 | alpha-2 | Non | Non |
| FGFDGDF | 0.94 | 57.56 | 7 | 803.91 | 0.1 | −0.21 | −2 | 3.56 | alpha-2 | Non | Non | |
| PAGPAGPIGPV | 0.83 | 20.76 | 11 | 932.24 | 0.18 | −0.39 | 0 | 5.96 | alpha-1 | Non | Non | |
| GPPGPMGPPGLA | 0.95 | 6.78 | 12 | 1047.41 | 0.11 | −0.3 | 0 | 5.52 | alpha-1 | Yes | Non | |
| PGPMGPSGPR | 0.87 | 3.71 | 10 | 952.23 | −0.16 | 0.2 | 1 | 10.18 | alpha-1 | Non | Non | |
| GPAGPAGPIGPVG | 0.90 | 2.54 | 13 | 1046.38 | 0.18 | −0.33 | 0 | 5.52 | alpha-1 | Non | Non | |
| GFDGDFY | 0.86 | 2.49 | 7 | 819.91 | 0.02 | −0.19 | −2 | 3.56 | alpha-2 | Non | Yes | |
| GPPGPMGPPGLAGPPGE | 0.91 | 1.94 | 17 | 1484.94 | 0.05 | −0.04 | −1 | 4.00 | alpha-1 | Yes | Non | |
| GPAGPAGPIGPV | 0.87 | 0.92 | 12 | 989.31 | 0.18 | −0.36 | 0 | 5.52 | alpha-1 | Non | Yes | |
| GPPGPMGPPGL | 0.97 | 0.87 | 11 | 976.32 | 0.1 | −0.28 | 0 | 5.52 | alpha-1 | Yes | Yes |
| Amino Acid Sequence | AHTP (Score) | ADP (Score) | AIP (Score) | FRS (Score) | CHEL (Score) | |
|---|---|---|---|---|---|---|
| AD | GGGPGPM | Yes (0.90) | Yes (0.83) | Yes (0.38) | 0.56 | 0.25 |
| GPPGPAGPAG | Yes (0.99) | Yes (0.89) | Yes (0.47) | 0.50 | 0.24 | |
| ADA | FGFDGDF | Non (0.12) | Yes (0.82) | Yes (0.44) | 0.42 | 0.24 |
| GPAGPAGPIGPVG | Yes (0.99) | Yes (0.94) | Yes (0.47) | 0.46 | 0.21 | |
| AGPAGPAGPAGPR | Yes (0.99) | Yes (0.59) | Yes (0.40) | 0.55 | 0.22 | |
| PGPMGPSGPR | Yes (0.95) | Yes (0.69) | Yes (0.36) | 0.49 | 0.23 | |
| PAGPAGPIGPV | Yes (0.98) | Yes (0.89) | Yes (0.48) | 0.47 | 0.22 |
| IC50 (mM) | Seq1 | Seq2 | Seq3 | Seq4 | Seq5 | Seq6 | Seq7 |
|---|---|---|---|---|---|---|---|
| ACE | 16.12 ± 2.11 a | 13.13 ± 1.63 ab | ND | 17.07 ± 2.45 a | 11.7 ± 1.34 b | 7.73 ± 0.54 c | 15.42 ± 2.08 a |
| DPP-IV | 1.26 ± 0.15 b | 0.82 ± 0.09 c | 0.59 ± 0.07 d | 0.07 ± 0.01 f | 4.56 ± 0.57 a | 3.74 ± 0.52 a | 0.24 ± 0.03 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Yang, Y.; Hu, B.; Zhu, L.; Liu, C.; Li, M.; Gu, Z.; Xin, Y.; Guo, Z.; Sun, H.; et al. The Bioaccessibility of Yak Bone Collagen Hydrolysates: Focus on Analyzing the Variation Regular of Peptides and Free Amino Acids. Foods 2023, 12, 1003. https://doi.org/10.3390/foods12051003
Guo Z, Yang Y, Hu B, Zhu L, Liu C, Li M, Gu Z, Xin Y, Guo Z, Sun H, et al. The Bioaccessibility of Yak Bone Collagen Hydrolysates: Focus on Analyzing the Variation Regular of Peptides and Free Amino Acids. Foods. 2023; 12(5):1003. https://doi.org/10.3390/foods12051003
Chicago/Turabian StyleGuo, Zitao, Yuliang Yang, Bo Hu, Lingyu Zhu, Chunyu Liu, Moying Li, Zhenghua Gu, Yu Xin, Zhongpeng Guo, Haiyan Sun, and et al. 2023. "The Bioaccessibility of Yak Bone Collagen Hydrolysates: Focus on Analyzing the Variation Regular of Peptides and Free Amino Acids" Foods 12, no. 5: 1003. https://doi.org/10.3390/foods12051003
APA StyleGuo, Z., Yang, Y., Hu, B., Zhu, L., Liu, C., Li, M., Gu, Z., Xin, Y., Guo, Z., Sun, H., Guan, Y., & Zhang, L. (2023). The Bioaccessibility of Yak Bone Collagen Hydrolysates: Focus on Analyzing the Variation Regular of Peptides and Free Amino Acids. Foods, 12(5), 1003. https://doi.org/10.3390/foods12051003