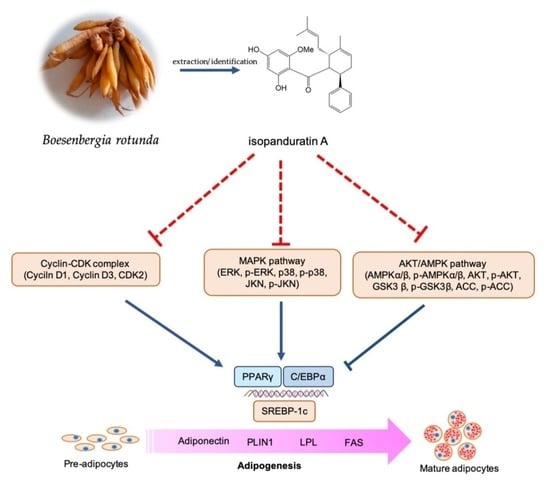
Inhibitory Effect of Isopanduratin A on Adipogenesis: A Study of Possible Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Culture Media
2.2. Cell Culture and Adipocyte Differentiation
2.3. Cytotoxicity Assay
2.4. Cell Proliferation Assay and Cell Cycle Analysis
2.5. Assessment of Cellular Lipid Content
2.6. Western Blotting
2.7. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statistical Analysis
3. Results
3.1. Effect of Isopanduratin A on Adipogenesis in 3T3-L1 Preadipocytes
3.2. Isopanduratin A Inhibits Mitotic Clonal Expansion during Adipogenesis
3.3. Isopanduratin A Downregulates Adipogenic Transcription Factors
3.4. Upstream Signals from MAPKs Are Modulated by Isopanduratin A
3.5. Isopanduratin A Modulates the Crosstalk between AMPK-ACC and AKT/GSK3β Signals
3.6. Isopanduratin A Suppresses Adipocyte Maturation in Human Preadipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 July 2022).
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A big picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Veerappan, K.; Ranjan, A.; Kim, Y.J.; Chellappan, D.K.; Dua, K.; Lee, J.; Perumalsamy, H. Phyllanthus emblica fruit extract attenuates lipid metabolism in 3T3-L1 adipocytes via activating apoptosis mediated cell death. Phytomedicine 2020, 66, 153129. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.T.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Petersen, R.K.; Sørensen, M.B.; Jørgensen, C.; Hallenborg, P.; Pridal, L.; Fleckner, J.; Amri, E.Z.; Krieg, P.; Furstenberger, G.; et al. Adipocyte differentiation of 3T3-L1 preadipocytes is dependent on lipoxygenase activity during the initial stages of the differentiation process. Biochem. J. 2003, 375, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ann, J.Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carling, D. The AMP-activated protein kinase cascade—A unifying system for energy control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.-L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.E.; Erickson, R.L.; Hemati, N.; MacDougald, O.A. Glycogen synthase kinase 3 is an insulin-regulated C/EBPα kinase. Mol. Cell. Biol. 1999, 19, 8433–8441. [Google Scholar] [CrossRef] [Green Version]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 1998, 273, 32111–32120. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Sato, F.; Fukunaga, H.; Iwasaki, Y.; Chiba, Y.; Tebakari, M.; Daigo, Y.; Kawashima, J.; Kamei, J. Placental extract suppresses differentiation of 3T3-L1 preadipocytes to mature adipocytes via accelerated activation of p38 MAPK during the early phase of adipogenesis. Nutr. Metab. 2019, 16, 32. [Google Scholar] [CrossRef]
- Munhoz, A.; Frode, T.S. Isolated Compounds from Natural Products with Potential Antidiabetic Activity—A Systematic Review. Curr. Diabetes Rev. 2018, 14, 36–106. [Google Scholar] [CrossRef]
- Qi, L.W.; Liu, E.H.; Chu, C.; Peng, Y.B.; Cai, H.X.; Li, P. Anti-diabetic agents from natural products—An update from 2004 to 2009. Curr. Top. Med. Chem. 2010, 10, 434–457. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Li-Ping, C.T.; Gen-Teck, F.; Khalid, N.; Abd Rahman, N.; Karsani, S.A.; et al. Boesenbergia rotunda: From Ethnomedicine to Drug Discovery. Evid.-Based Complement. Altern. Med. eCAM 2012, 2012, 473637. [Google Scholar] [CrossRef] [Green Version]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef]
- Chahyadi, A.; Hartati, R.; Wirasutisna, K.R. Boesenbergia pandurata Roxb., an Indonesian medicinal plant: Phytochemistry, biological activity, plant biotechnology. Procedia Chem. 2014, 13, 13–37. [Google Scholar] [CrossRef] [Green Version]
- Isa, N.; Abdelwahab, S.; Mohan, S.; Abdul, A.; Sukari, M.; Taha, M.; Syam, S.; Narrima, P.; Cheah, S.C.; Ahmad, S. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-Y.; Kim, M.-S.; Sa, B.-K.; Kim, M.-B.; Hwang, J.-K. Boesenbergia pandurata attenuates diet-induced obesity by activating AMP-activated protein kinase and regulating lipid metabolism. Int. J. Mol. Sci. 2012, 13, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Saah, S.; Siriwan, D.; Trisonthi, P. Biological activities of Boesenbergia rotunda parts and extracting solvents in promoting osteogenic differentiation of pre-osteoblasts. Food Biosci. 2021, 41, 101011. [Google Scholar] [CrossRef]
- Kirana, C.; Jones, G.P.; Record, I.R.; McIntoch, G.H. Anticancer properties of panduratin A isolated from Boesenbergia pandurata (Zingiberaceae). J. Nat. Med. 2007, 61, 131–137. [Google Scholar] [CrossRef]
- Ongwisespaiboon, O.; Jiraungkoorskul, W. Fingerroot, Boesenbergia rotunda and its Aphrodisiac Activity. Pharmacogn. Rev. 2017, 11, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. Interaction of panduratin A and derivatives with the SARS-CoV-2 main protease (mpro): A molecular docking study. J. Biomol. Struct. Dyn. 2022; 1–11, advance online publication. [Google Scholar] [CrossRef]
- Myoung, K.; Ahn, Y.T.; Lee, M.H.; Park, D.; Ahn, Y.M.; Huh, C.S. Fingerroot (Boesenbergia pandurata) Extract Inhibits the Accumulation of Visceral Fat in C57BL/6J Mice. J. Korean Soc. Food Sci. Nutr. 2013, 42, 26–32. [Google Scholar] [CrossRef]
- Chatsumpun, N.; Sritularak, B.; Likhitwitayawuid, K. New Biflavonoids with α-Glucosidase and Pancreatic Lipase Inhibitory Activities from Boesenbergia rotunda. Molecules 2017, 22, 1862. [Google Scholar] [CrossRef] [Green Version]
- San, H.T.; Khine, H.; Sritularak, B.; Prompetchara, E.; Chaotham, C.; Che, C.T.; Likhitwitayawuid, K. Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms. Foods 2022, 11, 3024. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, M.S.; Jo, K.; Lee, K.E.; Hwang, J.K. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARα/δ for the treatment of obesity. Diabetes Obes. Metab. 2011, 13, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Jin, H.; Lee, K.; Song, J.H.; Chei, S.; Oh, H.J.; Oh, J.H.; Lee, B.Y. Cardamonin suppresses lipogenesis by activating protein kinase A-mediated browning of 3T3-L1 cells. Phytomed. Int. J. Phytother. Phytopharm. 2019, 65, 153064. [Google Scholar] [CrossRef] [PubMed]
- Khine, H.E.E.; Sungthong, R.; Sritularak, B.; Prompetchara, E.; Chaotham, C. Untapped Pharmaceutical Potential of 4,5,4′-Trihydroxy-3,3′-dimethoxybibenzyl for Regulating Obesity: A Cell-Based Study with a Focus on Terminal Differentiation in Adipogenesis. J. Nat. Prod. 2022, 85, 1591–1602. [Google Scholar] [CrossRef]
- Yu, H.S.; Kim, W.J.; Bae, W.Y.; Lee, N.K.; Paik, H.D. Inula britannica Inhibits Adipogenesis of 3T3-L1 Preadipocytes via Modulation of Mitotic Clonal Expansion Involving ERK 1/2 and Akt Signaling Pathways. Nutrients 2020, 12, 3037. [Google Scholar] [CrossRef]
- Kim, W.J.; Yu, H.S.; Bae, W.Y.; Ko, K.Y.; Chang, K.H.; Lee, N.K.; Paik, H.D. Chrysanthemum indicum suppresses adipogenesis by inhibiting mitotic clonal expansion in 3T3-L1 preadipocytes. J. Food Biochem. 2021, 45, e13896. [Google Scholar] [CrossRef]
- Marcon, B.H.; Shigunov, P.; Spangenberg, L.; Pereira, I.T.; de Aguiar, A.M.; Amorín, R.; Rebelatto, C.K.; Correa, A.; Dallagiovanna, B. Cell cycle genes are downregulated after adipogenic triggering in human adipose tissue-derived stem cells by regulation of mRNA abundance. Sci. Rep. 2019, 9, 5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, M.; Rao, M.; Bouras, T.; Wang, C.; Wu, K.; Zhang, X.; Li, Z.; Yao, T.P.; Pestell, R.G. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment. J. Biol. Chem. 2005, 280, 16934–16941. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Bengoechea-Alonso, M.T.; Ericsson, J. A phosphorylation cascade controls the degradation of active SREBP1. J. Biol. Chem. 2009, 284, 5885–5895. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.J. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 896–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-Aguilar, F.; Pavillard, L.E.; Giampieri, F.; Bullón, P.; Cordero, M.D. Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. Int. J. Mol. Sci. 2017, 18, 288. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Yoneshiro, T.; Matsushita, M. Food Ingredients as Anti-Obesity Agents. Trends Endocrinol. Metab. TEM 2015, 26, 585–587. [Google Scholar] [CrossRef]
- Yao, Y.; Li, X.B.; Zhao, W.; Zeng, Y.Y.; Shen, H.; Xiang, H.; Xiao, H. Anti-obesity effect of an isoflavone fatty acid ester on obese mice induced by high fat diet and its potential mechanism. Lipids Health Dis. 2010, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef] [Green Version]
- Walton, R.G.; Zhu, B.; Unal, R.; Spencer, M.; Sunkara, M.; Morris, A.J.; Charnigo, R.; Katz, W.S.; Daugherty, A.; Howatt, D.A.; et al. Increasing adipocyte lipoprotein lipase improves glucose metabolism in high fat diet-induced obesity. J. Biol. Chem. 2015, 290, 11547–11556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Pu, Y.; Li, Z.H.; Liu, W.; Deng, Y.; Liang, R.; Zhang, X.M.; Zuo, H.D. Adiponectin, May Be a Potential Protective Factor for Obesity-Related Osteoarthritis. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 1305–1319. [Google Scholar] [CrossRef]
- Ranganathan, G.; Unal, R.; Pokrovskaya, I.; Yao-Borengasser, A.; Phanavanh, B.; Lecka-Czernik, B.; Rasouli, N.; Kern, P.A. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: Effects of obesity, insulin resistance, and TZD treatment. J. Lipid Res. 2006, 47, 2444–2450. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.; Jeon, M.; Kim, Y.S. Delphinidin, a major anthocyanin, inhibits 3T3-L1 pre-adipocyte differentiation through activation of Wnt/β-catenin signaling. BioFactors 2016, 42, 49–59. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol. Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.H.; Han, J.H.; Yu, K.H.; Hong, M.; Lee, S.Y.; Park, K.H.; Lee, S.U.; Kwon, T.H. Antioxidant and Anti-Obesity Activities of Polygonum cuspidatum Extract through Alleviation of Lipid Accumulation on 3T3-L1 Adipocytes. J. Microbiol. Biotechnol. 2020, 30, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2010, 425, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.D.; Xu, P.Z.; Chen, M.L.; Hahn-Windgassen, A.; Skeen, J.; Jacobs, J.; Sundararajan, D.; Chen, W.S.; Crawford, S.E.; Coleman, K.G. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003, 17, 1352–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS ONE 2013, 8, e70135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. TEM 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Huang, J.X.; Huang, H.Y.; Zhang, Y.Y.; Qian, S.W.; Zhu, H.; Zhang, Y.D.; Liu, Y.; Liu, Y.; et al. Histone demethylase Kdm4b functions as a co-factor of C/EBPβ to promote mitotic clonal expansion during differentiation of 3T3-L1 preadipocytes. Cell Death Differ. 2012, 19, 1917–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [Green Version]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmonte, N.; Phillips, B.W.; Massiera, F.; Villageois, P.; Wdziekonski, B.; Saint-Marc, P.; Nichols, J.; Aubert, J.; Saeki, K.; Yuo, A.; et al. Activation of extracellular signal-regulated kinases and CREB/ATF-1 mediate the expression of CCAAT/enhancer binding proteins beta and -delta in preadipocytes. Mol. Endocrinol. 2001, 15, 2037–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Targeted Gene | Primer | Nucleotide Sequence (5′-3′) |
|---|---|---|
| Pparg | PpargF | GATTCTCCTRTTGACCCAG |
| PpargR | GAR TGSGAGTGGTCTTCCAT | |
| C/ebpa | CebpaF | AGTCGGTGGACAAGAACAGC |
| CebpaR | GTGTCCAGTTCRCGGCTCA | |
| Srebp1c | Srebp1cF | YTGCMGACCCTGGTGAGTG |
| Srebp1cR | GASCGGTAGCGCTTCTCAAT | |
| Gadph | GADPHF | ACTCCACTCACGGCAAATTC |
| GADPHR | TCTCCATGGTGGTGAAGACA |
| Tested Chemical a | Relative Percentage of Oil Red O Stained Cells (%) b |
|---|---|
| Vehicle control c | 100.00 ± 0.00 |
| (1) Pinostrobin [C16H14O4] | 66.79 ± 2.34 * |
| (2) Geraniol [C10H18O] | 106.46 ± 3.34 |
| (3) Panduratin A [C26H30O4] | 76.94 ± 1.14 * |
| (4) Isopanduratin A [C26H30O4] | 64.04 ± 3.70 * |
| (5) Pinocembrin [C15H12O4] | 117.31 ± 7.05 |
| (6) Cardamonin [C16H14O4] | 80.89 ± 5.58 * |
| (7) Hydroxypanduratin A [C25H28O4] | 99.53 ± 0.59 |
| (8) 5,6-Dehydrokawain [C14H13O3] | 115.57 ± 2.89 |
| (9) Rotundaflavanochalcone [C31H26O8] | 115.05 ± 4.31 |
| (10) 2′,4′,6′-Trihydroxydihydrochalcone [C15H15O4] | 99.95 ± 3.92 |
| (11) Alpinetin [C16H15O4] | 108.18 ± 2.28 |
| (12) Iso-rotundaflavanochalcone [C31H26O8] | 86.31 ± 7.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungsa, P.; San, H.T.; Sritularak, B.; Böttcher, C.; Prompetchara, E.; Chaotham, C.; Likhitwitayawuid, K. Inhibitory Effect of Isopanduratin A on Adipogenesis: A Study of Possible Mechanisms. Foods 2023, 12, 1014. https://doi.org/10.3390/foods12051014
Rungsa P, San HT, Sritularak B, Böttcher C, Prompetchara E, Chaotham C, Likhitwitayawuid K. Inhibitory Effect of Isopanduratin A on Adipogenesis: A Study of Possible Mechanisms. Foods. 2023; 12(5):1014. https://doi.org/10.3390/foods12051014
Chicago/Turabian StyleRungsa, Prapenpuksiri, Htoo Tint San, Boonchoo Sritularak, Chotima Böttcher, Eakachai Prompetchara, Chatchai Chaotham, and Kittisak Likhitwitayawuid. 2023. "Inhibitory Effect of Isopanduratin A on Adipogenesis: A Study of Possible Mechanisms" Foods 12, no. 5: 1014. https://doi.org/10.3390/foods12051014
APA StyleRungsa, P., San, H. T., Sritularak, B., Böttcher, C., Prompetchara, E., Chaotham, C., & Likhitwitayawuid, K. (2023). Inhibitory Effect of Isopanduratin A on Adipogenesis: A Study of Possible Mechanisms. Foods, 12(5), 1014. https://doi.org/10.3390/foods12051014