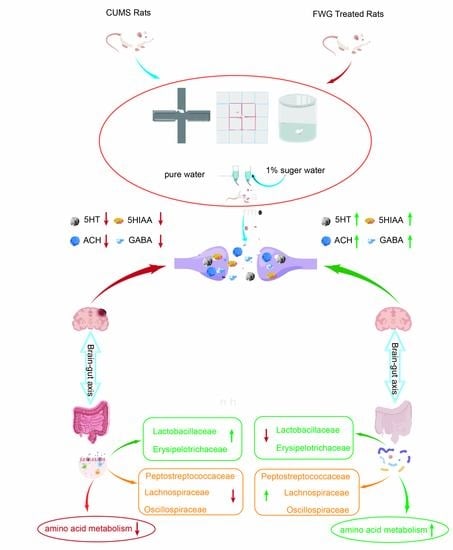
Fermented Wheat Germ Alleviates Depression-like Behavior in Rats with Chronic and Unpredictable Mild Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FWG
2.3. Animals
2.4. CUMS Procedure and Treatment
2.5. Elevated plus Maze Experiment
2.6. Forced Swimming Experiment
2.7. Open Field Test (OFT)
2.8. Sugar Preference Test (SPT)
2.9. Animal Handling and Tissue Dissection
2.10. Detection of Neurotransmitters
2.11. 16S rRNA Sequence Analysis of the Gut Microbiota
2.12. Statistical Analysis
3. Results
3.1. GABA Content in FWG Lyophilized Powder
3.2. Amelioration of CUMS-Induced Depression-like Behavior in Rats by FWG
3.3. Modulation of Neurotransmitters in the Hippocampus of CUMS Rats by FWG
3.4. Analysis of Gut Microbe Diversity
3.5. Analysis of Intestinal Flora Composition at Different Classification Levels
3.6. Correlation Analysis between Gut Microbiota and Neurotransmitters
3.7. Impact of FWG on Gut Microbiota Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-000510-5. [Google Scholar]
- Madhav, K.C.; Sherchand, S.P.; Sherchan, S. Association between screen time and depression among US adults. Prev. Med. Rep. 2017, 8, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, E.; Gramaglia, C.; Baldon, G.; Chirico, E.; Martelli, M.; Renolfi, A.; Zeppegno, P. “Gut–brain axis”: Review of the role of the probiotics in anxiety and depressive disorders. Brain Behav. 2020, 10, e01803. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Geng, W.; Pan, Y.; Wang, J.; Xiao, P.; Wang, Y. Supplementation with Lactobacillus kefiranofaciens ZW3 from Tibetan Kefir improves depression-like behavior in stressed mice by modulating the gut microbiota. Food Funct. 2019, 10, 925–937. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, P.; Tasleem, M.; Prakash, S.; Chouhan, G. Intermingling of gut microbiota with brain: Exploring the role of probiotics in battle against depressive disorders. Food Res. Int. 2020, 137, 109489. [Google Scholar] [CrossRef]
- Makris, A.P.; Karianaki, M.; Tsamis, K.I.; Paschou, S.A. The role of the gut-brain axis in depression: Endocrine, neural, and immune pathways. Hormones 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Aslam, H.; Green, J.; Jacka, F.N.; Collier, F.; Berk, M.; Pasco, J.; Dawson, S.L. Fermented foods, the gut and mental health: A mechanistic overview with implications for depression and anxiety. Nutr. Neurosci. 2018, 23, 659–671. [Google Scholar] [CrossRef]
- Yun, L.; Wang, S.; Wu, T.; Li, Q.; Zhang, M. Structural characterization of a novel glycoprotein in wheat germ and its physicochemical properties. Int. J. Biol. Macromol. 2018, 117, 1058–1065. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Probiotic Consumption Relieved Human Stress and Anxiety Symptoms Possibly via Modulating the Neuroactive Potential of the Gut Microbiota—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33511258/ (accessed on 21 October 2022).
- Dhaliwal, J.; Singh, D.P.; Singh, S.; Pinnaka, A.; Boparai, R.K.; Bishnoi, M.; Kondepudi, K.K.; Chopra, K. Lactobacillus plantarumMTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 2018, 125, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, Y.; Xu, F.; Cheng, Y.; Qi, W. Study on the fermentation condition for improving accumulation of γ-aminobutyric acid in fermented wheat germ by lactic acid bacteria. Food Ferment. Ind. 2014, 40, 77–82. (In Chinese) [Google Scholar] [CrossRef]
- Yanagisawa, K.; Moriyasu, F.; Miyahara, T.; Yuki, M.; Iijima, H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med. Biol. 2007, 33, 318–325. [Google Scholar] [CrossRef]
- Papp, M.; Moryl, E.; Willner, P. Pharmacological validation of the chronic mild stress model of depression. Eur. J. Pharmacol. 1996, 296, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Towell, A.; Sampson, D.; Sophokleous, S.; Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 1987, 93, 358–364. [Google Scholar] [CrossRef] [PubMed]
- O’Tuathaigh, C.M.; O’Connor, A.-M.; O’Sullivan, G.J.; Lai, D.; Harvey, R.; Croke, D.T.; Waddington, J.L. Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous ‘knockout’ of the schizophrenia risk gene neuregulin-1. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 462–466. [Google Scholar] [CrossRef]
- Porsolt, R.D.; LE Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Wu, C.; Yang, L.; Li, Y.; Dong, Y.; Yang, B.; Tucker, L.D.; Zong, X.; Zhang, Q. Effects of Exercise Training on Anxious–Depressive-like Behavior in Alzheimer Rat. Med. Sci. Sports Exerc. 2020, 52, 1456–1469. [Google Scholar] [CrossRef]
- Liu, C.; Ying, Z.; Li, Z.; Zhang, L.; Li, X.; Gong, W.; Sun, J.; Fan, X.; Yang, K.; Wang, X.; et al. Danzhi Xiaoyao Powder Promotes Neuronal Regeneration by Downregulating Notch Signaling Pathway in the Treatment of Generalized Anxiety Disorder. Front. Pharmacol. 2021, 12, 772576. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Ma, Y.; Li, X.; Zhang, J.; Zhao, L. Supplementation with soy isoflavones alleviates depression-like behaviour via reshaping the gut microbiota structure. Food Funct. 2021, 12, 4995–5006. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Shen, Y.; Huang, H.; Liu, Q.; Chen, C.; Ma, L.-H.; Wan, J.; Sun, Y.-Y.; Zhou, C.-H.; Wu, Y.-Q. Downregulated SIRT1 in the CeA is involved in chronic pain-depression comorbidity. Brain Res. Bull. 2021, 174, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-N.; Pan, L.; Liao, A.-M.; Hou, Y.-C.; Yu, G.-H.; Li, X.-X.; Yuan, Y.-J.; Dong, Y.-Q.; Zhang, Z.-S.; Tian, C.-Z.; et al. Wheat embryo globulin nutrients ameliorate d-galactose and aluminum chloride-induced cognitive impairment in rats. Brain Res. 2021, 1773, 147672. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.-H.; Peng, C.; Xie, X.-F.; Luo, M.; Zhu, H.; Feng, R.; Xiong, L. Alkaloids from the rhizomes of Ligusticum striatum exert antimigraine effects through regulating 5-HT1B receptor and c-Jun. J. Ethnopharmacol. 2019, 237, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Ge, H.; Sun, M.; Gao, Y. Selecting an Appropriate Animal Model of Depression. Int. J. Mol. Sci. 2019, 20, 4827. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Xu, J.; Lin, Y.; Ju, P.; Duan, D.; Luo, Y.; Ding, W.; Huang, S.; Chen, J.; Cui, D. Loss of Microglia and Impaired Brain-Neurotrophic Factor Signaling Pathway in a Comorbid Model of Chronic Pain and Depression. Front. Psychiatry 2018, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.-J.; Fan, S.-H.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Lv, M.; Wang, Y.; Qu, P.; Li, S.; Yu, Z.; Qin, X.; Liu, X. A combination of cecum microbiome and metabolome in CUMS depressed rats reveals the antidepressant mechanism of traditional Chinese medicines: A case study of Xiaoyaosan. J. Ethnopharmacol. 2021, 276, 114167. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Kim, K.-Y.; Jeong, H.-J.; Kim, H.-M. Antidepressant-like Effect of Altered Korean Red Ginseng in Mice. Behav. Med. 2011, 37, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Khanjani, S.; Moghaddam, A.H.; Braidy, N.; Nabavi, S.M. Improvement of Antioxidant Defences and Mood Status by Oral GABA Tea Administration in a Mouse Model of Post-Stroke Depression. Nutrients 2017, 9, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunello, N. The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur. Neuropsychopharmacol. 2002, 12, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M.; Felker, A. Monoamine Oxidase Inhibitors: A Modern Guide to an Unrequited Class of Antidepressants. CNS Spectr. 2008, 13, 855–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.-Y.; Shi, Y.-C.; You, H.-P.; Lo, Y.-H.; Pan, T.-M. Antidepressant Effect of GABA-RichMonascus-Fermented Product on Forced Swimming Rat Model. J. Agric. Food Chem. 2011, 59, 3027–3034. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [Green Version]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S. The gut microbiota in anxiety and depression – A systematic review. Clin. Psychol. Rev. 2020, 83, 101943. [Google Scholar] [CrossRef]
- Yu, L.; Han, X.; Cen, S.; Duan, H.; Feng, S.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef]
- Woo, J.K.; Choi, S.; Kang, J.-H.; Kim, D.E.; Hurh, B.-S.; Jeon, J.-E.; Kim, S.Y.; Oh, S.H. Fermented barley and soybean (BS) mixture enhances intestinal barrier function in dextran sulfate sodium (DSS)-induced colitis mouse model. BMC Complement. Altern. Med. 2016, 16, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Palm, N.W.; De Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A Coating Identifies Colitogenic Bacteria in Inflammatory Bowel Disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Bäuerl, C.; Collado, M.C.; Diaz Cuevas, A.; Viña, J.; Pérez Martínez, G. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 2018, 66, 464–471. [Google Scholar] [CrossRef]
- Evertsz’, F.B.; Thijssens, N.; Stokkers, P.; Grootenhuis, M.; Bockting, C.L.; Nieuwkerk, P.; Sprangers, M. Do Inflammatory Bowel Disease patients with anxiety and depressive symptoms receive the care they need? J. Crohn’s Colitis 2012, 6, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Zhu, Y.; Wu, W.; Zhang, Q.; Wang, Y.; Wang, Z.; Yang, F. A correlation study of intestinal microflora and first-episode depression in Chinese patients and healthy volunteers. Brain Behav. 2021, 11, e02036. [Google Scholar] [CrossRef]
- Yu, M.; Jia, H.; Zhou, C.; Yang, Y.; Zhao, Y.; Yang, M.; Zou, Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflam. 2021, 18, 254. [Google Scholar] [CrossRef]
- Xie, X.; Li, L.; Wu, X.; Hou, F.; Chen, Y.; Shi, L.; Liu, Q.; Zhu, K.; Jiang, Q.; Feng, Y.; et al. Alteration of the fecal microbiota in Chinese children with autism spectrum disorder. Autism Res. 2022, 15, 996–1007. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.-H.; Tung, Y.-T.; Lin, I.-H.; Shih, C.-K.; Nguyen, N.T.K.; Shabrina, A.; Huang, S.-Y. Fish Oil, but Not Olive Oil, Ameliorates Depressive-Like Behavior and Gut Microbiota Dysbiosis in Rats under Chronic Mild Stress. Biomolecules 2019, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Dunham, S.J.B.; McNair, K.A.; Adams, E.D.; Avelar-Barragan, J.; Forner, S.; Mapstone, M.; Whiteson, K.L. Longitudinal Analysis of the Microbiome and Metabolome in the 5xfAD Mouse Model of Alzheimer’s Disease. Mbio 2022, 13, e0179422. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, Y.; Li, X.; Jiang, J.; Kang, Y.; Pang, L.; Zhang, P.; Li, A.; Lv, L.; Andreassen, O.A.; et al. Gut microbial biomarkers for the treatment response in first-episode, drug-naïve schizophrenia: A 24-week follow-up study. Transl. Psychiatry 2021, 11, 422. [Google Scholar] [CrossRef]
- Qu, W.; Liu, S.; Zhang, W.; Zhu, H.; Tao, Q.; Wang, H.; Yan, H. Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress-induced depression-like behaviors: Intestinal microbiota and gut microbiome function. Food Funct. 2019, 10, 5886–5897. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Castells-Nobau, A.; Arnoriaga-Rodríguez, M.; Martin, M.; de la Vega-Correa, L.; Zapata, C.; Burokas, A.; Blasco, G.; Coll, C.; Escrichs, A.; et al. Microbiota alterations in proline metabolism impact depression. Cell Metab. 2022, 34, 681–701.e10. [Google Scholar] [CrossRef] [PubMed]
- Barbui, C.; Garattini, S. Tryptophan and depression. Lancet 1997, 349, 1553. [Google Scholar] [CrossRef]
- Vaiva, G.; Thomas, P.; Ducrocq, F.; Fontaine, M.; Boss, V.; Devos, P.; Rascle, C.; Cottencin, O.; Brunet, A.; Laffargue, P.; et al. Low posttrauma GABA plasma levels as a predictive factor in the development of acute posttraumatic stress disorder. Biol. Psychiatry 2004, 55, 250–254. [Google Scholar] [CrossRef]
- Mabunga, D.F.N.; Gonzales, E.L.T.; Kim, H.J.; Choung, S.Y. Treatment of GABA from Fermented Rice Germ Ameliorates Caffeine-Induced Sleep Disturbance in Mice. Biomol. Ther. 2015, 23, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A., Jr. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol. Psychiatry 2016, 81, 886–897. [Google Scholar] [CrossRef] [Green Version]








| Mass of FWG Dissolved in 1 mL of Water (mg/mL) | Absorbance (Measuring Tube) | Absorbance (Control Tube) | GABA Content in FWG (μg/g) |
|---|---|---|---|
| 1 mg | 0.218 | 0.209 | |
| 1 mg | 0.229 | 0.225 | |
| 1 mg | 0.237 | 0.224 | |
| 10 mg | 0.308 | 0.211 | 19,078.58 |
| 10 mg | 0.328 | 0.239 | 17,509.94 |
| 10 mg | 0.341 | 0.225 | 22,804.1 |
| 100 mg | 0.967 | 0.187 | |
| 100 mg | 0.869 | 0.212 | |
| 100 mg | 0.947 | 0.175 |
| Modules Number | Module Description Information |
|---|---|
| M00525 | Lysine biosynthesis, acetyl-DAP pathway |
| M00017 | Methionine biosynthesis, aspartate ≥ homoserine |
| M00026 | Histidine biosynthesis, PRPP ≥ histidine |
| M00570 | Isoleucine biosynthesis, threonine ≥ 2-oxobutanoate |
| M00023 | Tryptophan biosynthesis, chorismite ≥ tryptophan |
| M00609 | Cysteine biosynthesis, methionine ≥ cysteine |
| M00846 | Siroheme biosynthesis, glutamate ≥ siroheme |
| M00015 | Proline biosynthesis, glutamate ≥ proline |
| M00845 | Arginine biosynthesis, glutamate ≥ acetylcitrulline |
| M00028 | Ornithine biosynthesis, glutamate ≥ ornithine |
| M00025 | Tyrosine biosynthesis, chorismite ≥ tyrosine |
| M00024 | Phenylalanine biosynthesis |
| M00135 | GABA biosynthesis, eukaryotes, putrescine ≥ GABA |
| M00038 | Tryptophan metabolism, tryptophan≥ kynurenine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Zhao, P.; Liao, A.; Pan, L.; Zhang, J.; Dong, Y.; Huang, J.; He, W.; Ou, X. Fermented Wheat Germ Alleviates Depression-like Behavior in Rats with Chronic and Unpredictable Mild Stress. Foods 2023, 12, 920. https://doi.org/10.3390/foods12050920
Hu Z, Zhao P, Liao A, Pan L, Zhang J, Dong Y, Huang J, He W, Ou X. Fermented Wheat Germ Alleviates Depression-like Behavior in Rats with Chronic and Unpredictable Mild Stress. Foods. 2023; 12(5):920. https://doi.org/10.3390/foods12050920
Chicago/Turabian StyleHu, Zheyuan, Penghui Zhao, Aimei Liao, Long Pan, Jie Zhang, Yuqi Dong, Jihong Huang, Weiwei He, and Xingqi Ou. 2023. "Fermented Wheat Germ Alleviates Depression-like Behavior in Rats with Chronic and Unpredictable Mild Stress" Foods 12, no. 5: 920. https://doi.org/10.3390/foods12050920
APA StyleHu, Z., Zhao, P., Liao, A., Pan, L., Zhang, J., Dong, Y., Huang, J., He, W., & Ou, X. (2023). Fermented Wheat Germ Alleviates Depression-like Behavior in Rats with Chronic and Unpredictable Mild Stress. Foods, 12(5), 920. https://doi.org/10.3390/foods12050920