Assessment of Polyphenols Bioaccessibility, Stability, and Antioxidant Activity of White Mugwort (Artemisia lactiflora Wall.) during Static In Vitro Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
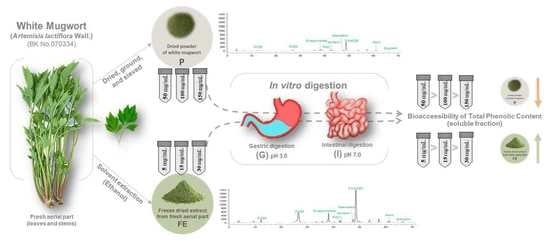
2.1.1. Preparation of Plant Materials
2.1.2. Quantification of Polyphenols in Original Starting Materials
2.2. Simulated Static In Vitro Gastrointestinal Digestion
2.2.1. Analysis of Total Phenolic Content (TPC)
2.2.2. Analysis of Antioxidant Capacity
2.2.3. Identification of Polyphenol Profile by HPLC-DAD/MS
2.3. Statistical Analysis
3. Results and Discussion
3.1. Changes in TPC and Antioxidant Activity of White Mugwort during In Vitro Gastrointestinal Digestion
3.2. Changes of Polyphenol Profile during In Vitro Gastrointestinal Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Ohto, Y.; Murakami, A.; Jiwajinda, S.; Ohigashi, H. Isolation and Identification of Acetylenic Spiroketal Enol Ethers from Artemisia lactiflora as Inhibitors of Superoxide Generation Induced by a Tumor Promoter in Differentiated HL-60 Cells. J. Agric. Food Chem. 1998, 46, 5031–5036. [Google Scholar] [CrossRef]
- Ma, L.; Ge, F.; Tang, C.-P.; Ke, C.-Q.; Li, X.-Q.; Althammer, A.; Ye, Y. The absolute configuration determination of naturally occurring diacetylenic spiroacetal enol ethers from Artemisia lactiflora. Tetrahedron 2011, 67, 3533–3539. [Google Scholar] [CrossRef]
- Xiao, M.-T.; Luo, D.-W.; Ke, Z.; Ye, J.; Tu, P.-F. A novel polyacetylene from the aerial parts of Artemisia lactiflora. Phytochem. Lett. 2014, 8, 52–54. [Google Scholar] [CrossRef]
- Lin, F.-D.; Luo, D.-W.; Ye, J.; Xiao, M.-T. Chemical constituents of Artemisia lactiflora(II). China J. Chin. Mater. Med. 2014, 39, 2531–2535. [Google Scholar]
- Namgoong, S.; Son, K.; Chang, H.; Kang, S.; Kim, H. Effects of naturally occurring flavonoids on mitogen-induced lymphocyte proliferation and mixed lymphocyte culture. Life Sci. 1994, 54, 313–320. [Google Scholar] [CrossRef]
- Kulprachakarn, K.; Pangjit, K.; Paradee, N.; Srichairatanakool, S.; Rerkasem, K.; Ounjaijean, S. Antioxidant Properties and Cytotoxicity of White Mugwort (Artemisia lactiflora) Leaf Extract in Human Hepatocellular Carcinoma Cell Line. Walailak J. Sci. Technol. (WJST) 2019, 16, 185–192. [Google Scholar] [CrossRef]
- Sea-tan, S.; Kunpanya, P. Water extract from leaf and stem of White Mugwort inhibits enzyme activity of α-amylase and α-glucosidase. J. Nutr. Assoc. Thail. 2017, 52, 16–22. [Google Scholar]
- Udomwasinakun, N.; Pirak, T.; Chanput, W.P. Identification of polyphenols in white mugwort (Artemisia lactiflora Wall.) ethanolic extracts and their anti-inflammatory and anti-adipogenic activity potential. Food Biosci. 2022, 47, 101761. [Google Scholar] [CrossRef]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.; Mira, L.; Corvo, M. Molecular Mechanisms of Anti-Inflammatory Activity Mediated by Flavonoids. Curr. Med. Chem. 2008, 15, 1586–1605. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Middleton, E., Jr. Comparison of the effects of quercetin with those of other flavonoids on the generation and effector function of cytotoxic T lymphocytes. Immunopharmacology 1984, 7, 115–126. [Google Scholar] [CrossRef]
- Khalilpourfarshbafi, M.; Gholami, K.; Murugan, D.D.; Sattar, M.Z.A.; Abdullah, N.A. Differential effects of dietary flavonoids on adipogenesis. Eur. J. Nutr. 2018, 58, 5–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Kogami, Y.; Nakamura, S.; Sugiyama, T.; Ueno, T.; Yoshikawa, M. Structural requirements of flavonoids for the adipogenesis of 3T3-L1 cells. Bioorg. Med. Chem. 2011, 19, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Narita, Y.; Inouye, K. Degradation Kinetics of Chlorogenic Acid at Various pH Values and Effects of Ascorbic Acid and Epigallocatechin Gallate on Its Stability under Alkaline Conditions. J. Agric. Food Chem. 2013, 61, 966–972. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Oliveira, A.; Jesus, D.; Rodrigues, C.; Figueira, C.; Gomes, A.; Pintado, M. Chlorogenic acids composition and the impact of in vitro gastrointestinal digestion on espresso coffee from single-dose capsule. Food Res. Int. 2020, 134, 109223. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Czubinski, J.; Wroblewska, K.; Czyzniejewski, M.; Górnaś, P.; Kachlicki, P.; Siger, A. Bioaccessibility of defatted lupin seed phenolic compounds in a standardized static in vitro digestion system. Food Res. Int. 2018, 116, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Kim, Y.E.; Ryu, J.; Kim, J.T.; Suh, S.; Hong, G.-P.; Ko, S. Physicochemical and in vitro digestion characteristics of size-different red ginseng powders. Food Sci. Biotechnol. 2017, 27, 425–431. [Google Scholar] [CrossRef]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.-L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.-H.; Lee, C.-Y.; Lee, Y.-E.; Yoo, S.-H.; Chung, J.-O.; Rha, C.-S.; Park, M.-Y.; Hong, Y.-D.; Shim, S.-M. Profiling of In Vitro Bioaccessibility and Intestinal Uptake of Flavonoids after Consumption of Commonly Available Green Tea Types. Molecules 2021, 26, 1518. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, Z.; Dai, M.; Cao, X.; Xiong, X.; He, S.; Yuan, Y.; Zhang, M.; Dong, L.; Zhang, R.; et al. Effects of simulated digestion on the phenolic composition and antioxidant activity of different cultivars of lychee pericarp. BMC Chem. 2019, 13, 27. [Google Scholar] [CrossRef]
- Daly, T.; Jiwan, M.A.; O’Brien, N.M.; Aherne, S.A. Carotenoid Content of Commonly Consumed Herbs and Assessment of Their Bioaccessibility Using an In Vitro Digestion Model. Plant Foods Hum. Nutr. 2010, 65, 164–169. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E. In vitro gastrointestinal digestion of polyphenols from different molasses (pekmez) and leather (pestil) varieties. Int. J. Food Sci. Technol. 2013, 49, 1027–1039. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Soković Bajić, S.; Jovanović, Ž.; Matić, I.; Tolinački, M.; Popović, D.; Popović, N.; Terzić-Vidojević, A.; Golić, N.; Beškoski, V.; et al. Antioxidant and Antiproliferative Activity of Allium ursinum and Their Associated Microbiota During Simulated in vitro Digestion in the Presence of Food Matrix. Front. Microbiol. 2020, 11, 601616. [Google Scholar] [CrossRef]
- Gião, M.S.; Gomes, S.; Madureira, A.R.; Faria, A.; Pestana, D.; Calhau, C.; Pintado, M.E.; Azevedo, I.; Malcata, F.X. Effect of in vitro digestion upon the antioxidant capacity of aqueous extracts of Agrimonia eupatoria, Rubus idaeus, Salvia sp. and Satureja montana. Food Chem. 2012, 131, 761–767. [Google Scholar] [CrossRef]
- Gonçalves, G.A.S.; Resende, N.S.; Carvalho, E.E.N.; de Resende, J.V.; Boas, E.V.D.B.V. Effect of pasteurisation and freezing method on bioactive compounds and antioxidant activity of strawberry pulp. Int. J. Food Sci. Nutr. 2017, 68, 682–694. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; León-Félix, J.; Heredia, J.B. Effect of In Vitro Digestion on the Total Antioxidant Capacity and Phenolic Content of 3 Species of Oregano (Hedeoma patens, Lippia graveolens, Lippia palmeri). J. Food Sci. 2017, 82, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wan, L.; Wu, C.; Fang, Y.; Han, G.; Li, H.; Zhang, Z.; Wang, H. Simultaneous Determination of 14 Phenolic Compounds in Grape Canes by HPLC-DAD-UV Using Wavelength Switching Detection. Molecules 2013, 18, 14241–14257. [Google Scholar] [CrossRef] [Green Version]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of in vitro digestion on bioactive compounds, antioxidant and antimicrobial activities of coffee (Coffea arabica L.) pulp aqueous extract. Food Chem. 2021, 348, 129094. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Vidal, J.; Ruiz-Riaguas, A.; Córdova, M.F.-D.; Ortega-Barrales, P.; Llorent-Martínez, E. Phenolic profile and antioxidant activity of Jasonia glutinosa herbal tea. Influence of simulated gastrointestinal in vitro digestion. Food Chem. 2019, 287, 258–264. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Campoli, S.S.; Rojas, M.L.; Amaral, J.E.P.G.D.; Canniatti-Brazaca, S.G.; Augusto, P.E.D. Ultrasound processing of guava juice: Effect on structure, physical properties and lycopene in vitro accessibility. Food Chem. 2018, 268, 594–601. [Google Scholar] [CrossRef]
- Karabegović, I.; Nikolova, M.; Veličković, D.; Stojičević, S.; Veljković, V.; Lazić, M. Comparison of Antioxidant and Antimicrobial Activities of Methanolic Extracts of the Artemisia sp. Recovered by Different Extraction Techniques. Chin. J. Chem. Eng. 2011, 19, 504–511. [Google Scholar] [CrossRef]
- Hwang, K.-E.; Choi, Y.-S.; Choi, J.-H.; Kim, H.-Y.; Kim, H.-W.; Lee, M.-A.; Chung, H.-K.; Kim, C.-J. Effect of Ganghwayakssuk (Artemisia princeps Pamp.) on oxidative stability of deep fried chicken nuggets. Food Sci. Biotechnol. 2011, 20, 1381–1388. [Google Scholar] [CrossRef]
- Ketnawa, S.; Suwannachot, J.; Ogawa, Y. In vitro gastrointestinal digestion of crisphead lettuce: Changes in bioactive compounds and antioxidant potential. Food Chem. 2019, 311, 125885. [Google Scholar] [CrossRef]
- Sivapalan, T.; Melchini, A.; Coode-Bate, J.; Needs, P.W.; Mithen, R.F.; Saha, S. An LC-MS/MS Method to Measure S-Methyl-l-Cysteine and S-Methyl-l-Cysteine Sulfoxide in Human Specimens Using Isotope Labelled Internal Standards. Molecules 2019, 24, 2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennouna, D.; Avice, J.-C.; Rosique, C.; Svilar, L.; Pontet, C.; Trouverie, J.; Fine, F.; Pinochet, X.; Fraser, K.; Martin, J.-C. The impact of genetics and environment on the polar fraction metabolome of commercial Brassica napus seeds: A multi-site study. Seed Sci. Res. 2019, 29, 167–178. [Google Scholar] [CrossRef]
- von Roepenack-Lahaye, E.; Degenkolb, T.; Zerjeski, M.; Franz, M.; Roth, U.; Wessjohann, L.; Schmidt, J.; Scheel, D.; Clemens, S. Profiling of Arabidopsis Secondary Metabolites by Capillary Liquid Chromatography Coupled to Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. Plant Physiol. 2004, 134, 548–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nignpense, B.E.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. The impact of simulated gastrointestinal digestion on the bioaccessibility and antioxidant activity of purple rice phenolic compounds. Food Biosci. 2022, 47, 101706. [Google Scholar] [CrossRef]
- Yang, I.; Jayaprakasha, G.K.; Patil, B. In vitro digestion with bile acids enhances the bioaccessibility of kale polyphenols. Food Funct. 2018, 9, 1235–1244. [Google Scholar] [CrossRef]
- Durdun, C.; Papuc, C.; Nicorescu, V.; Gajaila, I.; Goran, G.; Petcu, C.; Stefan, G. The Influence of Solid-to-Solvent Ratio and Extraction Method on Total Phenolic Content, Flavonoid Content and Antioxidant Properties of Some Ethanolic Plant Extracts. Rev. Chim. 2016, 67, 1922–1927. [Google Scholar]
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The effect of in vitro digestion, food matrix, and hydrothermal treatment on the potential bioaccessibility of selected phenolic compounds. Food Chem. 2020, 344, 128581. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Qin, Y.; Liu, J.; Liu, J.; Wang, Q.; Ren, F.; Zhang, H. Interaction of phenolic acids and their derivatives with human serum albumin: Structure–affinity relationships and effects on antioxidant activity. Food Chem. 2018, 240, 1072–1080. [Google Scholar] [CrossRef]
- Li, D.-L.; Zheng, X.-L.; Duan, L.; Deng, S.-W.; Ye, W.; Wang, A.-H.; Xing, F.-W. Ethnobotanical survey of herbal tea plants from the traditional markets in Chaoshan, China. J. Ethnopharmacol. 2017, 205, 195–206. [Google Scholar] [CrossRef]
- Liang, Y.; Abbott, D.; Howard, N.; Lim, K.; Ward, R.; Elgendi, M. How Effective Is Pulse Arrival Time for Evaluating Blood Pressure? Challenges and Recommendations from a Study Using the MIMIC Database. J. Clin. Med. 2019, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Wahyuni, T.S.; Purwanto, K.K. Students’ conceptual understanding on acid-base titration and its relationship with drawing skills on a titration curve. J. Phys. Conf. Ser. 2020, 1440, 012018. [Google Scholar] [CrossRef]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 873–886. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Francenia Santos Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. Antioxidants 2019, 22, 1–29. [Google Scholar]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive Phenolics of the Genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS Profile of the Siberian Species and Their Inhibitory Potential Against α-Amylase and α-Glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.-Y.; Lin, S.-Y.; Lin, K.-H.; Liu, Y.-F.; Hsu, J.-I.; Yang, C.-M.; Lai, J.-Y. Antioxidant Activity in Extracts of 27 Indigenous Taiwanese Vegetables. Nutrients 2014, 6, 2115–2130. [Google Scholar] [CrossRef] [Green Version]
- Booth, A.N.; Emerson, O.; Jones, F.T.; DeEds, F. Urinary metabolites of caffeic and chlorogenic acids. J. Biol. Chem. 1957, 229, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.N.; Williams, R.T. Dehydroxylation of Caffeic Acid by Rat and Rabbit Cæcal Contents and Sheep Rumen Liquor. Nature 1963, 198, 684–685. [Google Scholar] [CrossRef]





| Compounds (mg/g DW) | White Mugwort Powder (P) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-MetOH | P 50 mg/mL | P 100 mg/mL | P 150 mg/mL | ||||||||||
| Gastric | R (%) | Intestinal | BA (%) | Gastric | R (%) | Intestinal | BA (%) | Gastric | R (%) | Intestinal | BA (%) | ||
| Quinic acid | ND | 0.2 ± 0 B | - | 0.8 ± 0 A | - | 0.05 ± 0 B | - | 0.25 ± 0 B | - | 0.2 ± 0.0 B | - | 0.2 ± 0 B | - |
| 5-CQA | 0.04 ± 0 ns | 0.04 ± 0 ns | 88.8 | ND | 0 | 0.03 ± 0 ns | 87.2 | ND | 0 | 0.04 ± 0 ns | 96.3 | ND | 0.0 |
| 3-CQA | 1.4 ± 0.1 A | 0.14 ± 0 C | 9.7 | ND | 0 | 0.4 ± 0 BC | 30.1 | ND | 0 | 0.6 ± 0.0 B | 39.0 | ND | 0.0 |
| Rutin | 0.6 ± 0.0 A | 0.2 ± 0 B | 32.0 | 0.2 ± 0 B | 35 | 0.1 ± 0 B | 20.7 | 0.02 ± 0 B | 32 | 0.1 ± 0.0 B | 18.0 | 0.3 ± 0 B | 39 |
| Isovitexin | 0.4 ± 0.0 A | 0.1 ± 0 B | 38.0 | ND | 0 | 0.1 ± 0 C | 21.1 | ND | 0 | 0.1 ± 0.0 C | 18.0 | ND | 0.0 |
| Kaempferol | 1.2 ± 0.1 A | ND | 0 | ND | 0 | ND | 0 | ND | 0 | 0.2 ± 0.0 B | 18.2 | 0.02 ± 0 C | 1.5 |
| 3,5-diCQA | 4.6 ± 0.2 A | 1.0 ± 0 B | 21.5 | ND | 0 | 0.5 ± 0 C | 10.7 | 0.1 ± 0 D | 1.5 | 0.4 ± 0 CD | 9.6 | 0.1 ± 0 D | 1.4 |
| Morin | 0.6 ± 0.1 A | 0.13 ± 0 B | 21.0 | ND | 0 | 0.1 ± 0 BC | 18.5 | ND | 0 | 0.04 ± 0 CD | 7.2 | 0.1 ± 0 BC | 11 |
| Quercetin | 0.1 ± 0.0 A | 0.03 ± 0 B | 26.4 | ND | 0 | 0.03 ± 0 B | 27.6 | 0.03 ± 0 B | 25 | 0.03 ± 0 B | 25.3 | 0.04 ± 0 B | 31 |
| Compounds (mg/g DW) | White Mugwort Extract (FE) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FE-MetOH | FE 5 mg/mL | FE 15 mg/mL | FE 30 mg/mL | ||||||||||
| Gastric | R (%) | Intestinal | BA (%) | Gastric | R (%) | Intestinal | BA (%) | Gastric | R (%) | Intestinal | BA (%) | ||
| Quinic acid | ND | ND | - | 6.1 ± 0 A | - | 0.1 ± 0.0 C | - | 1.7 ± 0.6 B | - | ND | - | 0.7 ± 0.2 BC | - |
| 5-CQA | 0.3 ± 0.0 B | 0.2 ± 0.0 B | 80.6 | 4.3 ± 0 A | 1671 | 0.3 ± 0.0 B | 125 | ND | 0 | 0.4 ± 0.1 B | 150 | ND | 0.00 |
| 3-CQA | 22.3 ± 2.3 A | 11 ± 3.9 CD | 50.5 | ND | 0 | 17 ± 1.0 B | 77.5 | 8 ± 1. D | 36.0 | 20 ± 2.3 AB | 90.6 | 13.0 ± 0 C | 58.4 |
| Rutin | 1.8 ± 0.0 B | 2.1 ± 0.2 A | 122 | ND | 0 | 1.0 ± 0.1 D | 55.6 | 1.2 ± 0 CD | 68.5 | 1.3 ± 0.1 C | 73.9 | 1.0 ± 0.1 D | 56.2 |
| Isovitexin | 0.9 ± 0.0 B | 1.5 ± 0.4 A | 167 | ND | 0 | 0.6 ± 0.2 C | 60.8 | ND | 0 | 0.7 ± 0.1 BC | 80.1 | 0.4 ± 0.0 C | 46.9 |
| Kaempferol | 5.3 ± 1.0 B | ND | 0 | ND | 0 | 11.2 ± 5.7 A | 210 | 1.0 ± 0.2 B | 18.8 | 13.3 ± 5.7 A | 249 | 5.3 ± 0.2 B | 99.0 |
| 3,5-diCQA | 19.9 ± 6.1 A | 19 ± 7.0 AB | 95.3 | 1.0 ± 0 C | 3.9 | 14.2 ± 2.6 B | 71.6 | 0.9 ± 0.1 C | 4.3 | 16 ± 4.4 AB | 82.2 | 3.1 ± 0.1 C | 15.7 |
| Morin | 2.2 ± 0.1 A | 1.1 ± 0.2 BC | 52.6 | ND | 0 | 1.2 ± 0.8 BC | 52.9 | 0.6 ± 0.1 C | 25.4 | 1.7 ± 0.3 AB | 76.4 | 0.6 ± 0.1 C | 29.1 |
| Quercetin | 0.7 ± 0.0 AB | 0.7 ± 0.2 A | 106 | ND | 0 | 0.3 ± 0 BC | 40.5 | 0.3 ± 0 AB | 51.8 | 0.4 ± 0.1 AB | 53.1 | 0.1 ± 0.0 C | 12.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udomwasinakun, N.; Saha, S.; Mulet-Cabero, A.-I.; Wilde, P.J.; Pirak, T. Assessment of Polyphenols Bioaccessibility, Stability, and Antioxidant Activity of White Mugwort (Artemisia lactiflora Wall.) during Static In Vitro Gastrointestinal Digestion. Foods 2023, 12, 949. https://doi.org/10.3390/foods12050949
Udomwasinakun N, Saha S, Mulet-Cabero A-I, Wilde PJ, Pirak T. Assessment of Polyphenols Bioaccessibility, Stability, and Antioxidant Activity of White Mugwort (Artemisia lactiflora Wall.) during Static In Vitro Gastrointestinal Digestion. Foods. 2023; 12(5):949. https://doi.org/10.3390/foods12050949
Chicago/Turabian StyleUdomwasinakun, Nacha, Shikha Saha, Ana-Isabel Mulet-Cabero, Peter James Wilde, and Tantawan Pirak. 2023. "Assessment of Polyphenols Bioaccessibility, Stability, and Antioxidant Activity of White Mugwort (Artemisia lactiflora Wall.) during Static In Vitro Gastrointestinal Digestion" Foods 12, no. 5: 949. https://doi.org/10.3390/foods12050949
APA StyleUdomwasinakun, N., Saha, S., Mulet-Cabero, A. -I., Wilde, P. J., & Pirak, T. (2023). Assessment of Polyphenols Bioaccessibility, Stability, and Antioxidant Activity of White Mugwort (Artemisia lactiflora Wall.) during Static In Vitro Gastrointestinal Digestion. Foods, 12(5), 949. https://doi.org/10.3390/foods12050949