Impact of Different Drying Methods on the Phenolic Composition, In Vitro Antioxidant Activity, and Quality Attributes of Dragon Fruit Slices and Pulp
Abstract
:1. Introduction
2. Materials and Methods
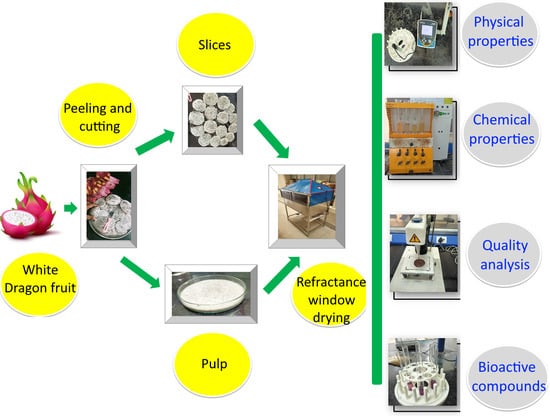
2.1. Sample Preparation
2.2. Operating Conditions of Drying
2.3. Procedure of Preparing a Methanolic Extract Solution
2.4. Determination of Quality Parameters
2.4.1. Moisture Content
2.4.2. Texture Evaluation
2.4.3. Color Analysis
2.4.4. pH, Total Ash Content, and Total Soluble Solids (TSS)
2.4.5. Crude Fiber Content
2.4.6. TPC
2.4.7. TFC
2.4.8. DPPH Radical Scavenging Activity
2.4.9. FTIR Spectra
2.5. Statistical Analysis
3. Results
3.1. Moisture Content (MC)
3.2. Drying Characteristics of Dragon Fruit Sample
3.3. pH, Ash Content, TSS, Crude Fiber, and Drying Time
3.4. Texture Analysis
3.5. Color Analysis
3.6. TPC, TFC, and AA
3.7. FTIR Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raj, G.B.; Dash, K.K. Microwave vacuum drying of dragon fruit slice: Artificial neural network modelling, genetic algorithm optimization, and kinetics study. Comput. Electron. Agric. 2020, 178, 105814. [Google Scholar] [CrossRef]
- Matan, N.; Puangjinda, K.; Phothisuwan, S.; Nisoa, M. Combined antibacterial activity of green tea extract with atmospheric radio-frequency plasma against pathogens on fresh-cut dragon fruit. Food Control 2015, 50, 291–296. [Google Scholar] [CrossRef]
- Jalgaonkar, K.; Mahawar, M.K.; Bibwe, B.; Kannaujia, P. Postharvest Profile, Processing and Waste Utilization of Dragon Fruit (Hylocereus Spp.): A Review. Food Rev. Int. 2020, 38, 733–759. [Google Scholar] [CrossRef]
- Jaafar, R.A.; Ridhwan, A.; Abdul, B.; Naning, T.; Gajah, A. Proximate Analysis of Dragon Fruit (Hylecereus polyhizus). Am. J. Appl. Sci. 2009, 6, 1341–1346. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Martínez, C.; Quintero, P.; Ayala, A.; Ortiz, M. Drying characteristics of mango slices using the Refractance Window™ technique. J. Food Eng. 2012, 109, 69–75. [Google Scholar] [CrossRef]
- Spolmann, O.; Nocetti, D.; Zura-bravo, L.; Lemus-mondaca, R. Effects of Infrared-Assisted Refractance Window™ Drying on the Drying Kinetics, Microstructure, and Color of Physalis Fruit Purée. Foods 2020, 9, 343. [Google Scholar]
- Ladha-Sabur, A.; Bakalis, S.; Fryer, P.J.; Lopez-Quiroga, E. Mapping energy consumption in food manufacturing. Trends Food Sci. Technol. 2019, 86, 270–280. [Google Scholar] [CrossRef]
- Baeghbali, V.; Niakousari, M.; Farahnaky, A. Refractance Window drying of pomegranate juice: Quality retention and energy efficiency. LWT 2016, 66, 34–40. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E.; Özdemir, M.; Topuz, A. Quality characteristics of bone broth powder obtained through Refractance Window™ drying. LWT 2021, 147, 111526. [Google Scholar] [CrossRef]
- Puente, L.; Vega-Gálvez, A.; Ah-Hen, K.S.; Rodríguez, A.; Pasten, A.; Poblete, J.; Pardo-Orellana, C.; Muñoz, M. Refractance Window drying of goldenberry (Physalis peruviana L.) pulp: A comparison of quality characteristics with respect to other drying techniques. LWT 2020, 131, 109772. [Google Scholar] [CrossRef]
- Santos, S.D.J.L.; Canto, H.K.F.; da Silva, L.H.M.; Rodrigues, A.M.D.C. Characterization and properties of purple yam (Dioscorea trifida) powder obtained by refractance window drying. Dry. Technol. 2020, 40, 1103–1113. [Google Scholar] [CrossRef]
- Jalgaonkar, K.; Mahawar, M.K.; Vishwakarma, R.K.; Shivhare, U.S.; Nambi, V.E. Optimization of process condition for preparation of sapota bar using Refractance window drying method. Dry. Technol. 2018, 38, 269–278. [Google Scholar] [CrossRef]
- Ayala-Aponte, A.; Cárdenas-Nieto, J.; Tirado, D. Aloe vera Gel Drying by Refractance Window®: Drying Kinetics and High-Quality Retention. Foods 2021, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Tontul, I.; Eroğlu, E.; Topuz, A. Convective and refractance window drying of cornelian cherry pulp: Effect on physicochemical properties. J. Food Process. Eng. 2018, 41, e12917. [Google Scholar] [CrossRef]
- Tontul, I.; Ergin, F.; Eroğlu, E.; Küçükçetin, A.; Topuz, A. Physical and microbiological properties of yoghurt powder produced by refractance window drying. Int. Dairy J. 2018, 85, 169–176. [Google Scholar] [CrossRef]
- Asiimwe, A.; Kigozi, J.; Baidhe, E.; Muyonga, J. Optimization of refractance window drying conditions for passion fruit puree. LWT 2022, 154, 112742. [Google Scholar] [CrossRef]
- Bernaert, N.; Van Droogenbroeck, B.; Van Pamel, E.; De Ruyck, H. Innovative refractance window drying technology to keep nutrient value during processing. Trends Food Sci. Technol. 2019, 84, 22–24. [Google Scholar] [CrossRef]
- Nindo, C.I.; Tang, J. Refractance Window Dehydration Technology: A Novel Contact Drying Method. Dry. Technol. 2007, 25, 37–48. [Google Scholar] [CrossRef]
- Jafari, S.-M.; Azizi, D.; Mirzaei, H.; Dehnad, D. Comparing Quality Characteristics of Oven-Dried and Refractance Window-Dried Kiwifruits. J. Food Process. Preserv. 2016, 40, 362–372. [Google Scholar] [CrossRef]
- Trivedi, M.; D’Costa, V.; Shitut, J.; Srivastav, S. Refractance window technology—A Promising Drying Technique for the Food Industry. J. Innov. Emerg. Res. Eng. 2017, 4, 6–16. [Google Scholar]
- Ortiz-Jerez, M.J.; Gulati, T.; Datta, A.K.; Ochoa-Martínez, C.I. Quantitative understanding of Refractance Window™ drying. Food Bioprod. Process. 2015, 95, 237–253. [Google Scholar] [CrossRef]
- Karadbhajne, S.V.; Thakare, V.M.; Kardile, N.B.; Thakre, S.M. Refractance Window Drying: An Innovative Drying Technique for Heat Sensitive Product. Int. J. Recent Technol. Eng. 2019, 8, 1765–1771. [Google Scholar] [CrossRef]
- Zotarelli, M.F.; Carciofi, B.A.M.; Laurindo, J.B. Effect of process variables on the drying rate of mango pulp by Refractance Window. Food Res. Int. 2015, 69, 410–417. [Google Scholar] [CrossRef]
- Maduwanthi, S.; Marapana, R. Total phenolics, flavonoids and antioxidant activity following simulated gastro-intestinal digestion and dialysis of banana (Musa acuminata, AAB) as affected by induced ripening agents. Food Chem. 2021, 339, 127909. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis of the Association of Official Analytical Chemists; The Association: Arlington, VA, USA, 2000; Volume 17. [Google Scholar]
- Kek, S.; Chin, N.; Yusof, Y. Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food Bioprod. Process. 2013, 91, 495–506. [Google Scholar] [CrossRef]
- Nayak, P.K.; Mohan, C.C.; Radhakrishnan, K. Effect of microwave pretreatment on the color degradation kinetics in mustard greens (Brassica juncea). Chem. Eng. Commun. 2018, 205, 1261–1273. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of total phenolic content using the Folin-C assay: Single-laboratory validation, first action 2017.13. J. AOAC Int. 2019, 102, 320–321. [Google Scholar] [CrossRef] [Green Version]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.M.; Deshpande, N.R.; Kashalkar, R.V. Spectroscopic determination of total phenol and flavonoid contents of ipomoea carnea. Int. J. ChemTech Res. 2010, 2, 1698–1701. [Google Scholar]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Phytochemical content and antioxidant activities of thirteen fruits of Assam, India. Food Biosci. 2016, 13, 15–20. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Chen, J.; Ao, Q. FTIR, XRD and SEM Analysis of Ginger Powders with Different Size. J. Food Process. Preserv. 2015, 39, 2017–2026. [Google Scholar] [CrossRef]
- Liu, Y.; Dave, D.; Trenholm, S.; Ramakrishnan, V.; Murphy, W. Effect of Drying on Nutritional Composition of Atlantic Sea Cucumber (Cucumaria frondosa) Viscera Derived from Newfoundland Fisheries. Processes 2021, 9, 703. [Google Scholar] [CrossRef]
- Krishnan, K.R.; Rayaguru, K.; Nayak, P.K. Ultra-sonicated vacuum drying’s effect on antioxidant activity, TPC, TFC and color of elephant apple slices. Food Biosci. 2020, 36, 100629. [Google Scholar] [CrossRef]
- Dadhaneeya, H.; Nayak, P.K.; Saikia, D.; Kondareddy, R.; Ray, S.; Kesavan, R.K. The impact of refractance window drying on the physicochemical properties and bioactive compounds of malbhog banana slice and pulp. Appl. Food Res. 2023, 3, 100279. [Google Scholar] [CrossRef]
- Abirami, K.; Swain, S.; Baskaran, V.; Venkatesan, K.; Sakthivel, K.; Bommayasamy, N. Distinguishing three Dragon fruit (Hylocereus spp.) species grown in Andaman and Nicobar Islands of India using morphological, biochemical and molecular traits. Sci. Rep. 2021, 11, 2894. [Google Scholar] [CrossRef]
- Rahman, N.F.A.; Ismail, A.; Shah, N.N.A.K.; Varith, J.; Shamsudin, R. Effect of drying temperature on Malaysia pomelo (Citrus grandis (L.) osbeck) pomace residue under vacuum condition. Pertanika J. Sci. Technol. 2019, 27, 57–66. [Google Scholar]
- Adeboye, O.A.; Iyanda, R.A.; Yusuf, K.A.; Olaniyan, A.M.; Oje, K.O. Effects of temperature, pre-treatment and slice orientation on the drying rate and post drying qualities of green plantain (Musa paradisiaca). Int. J. Technol. Enhanc. Emerg. Eng. Res. 2014, 2, 2347–4289. [Google Scholar]
- Ho, L.-H.; Suhaimi, A.; Ismail, I.; Mustafa, A. Effect of Different Drying Conditions on Proximate Compositions of Red-and Yellow-Fleshed Watermelon Rind Powders. J. Agrobiotech. 2016, 7, 1–12. [Google Scholar]
- Kondareddy, R.; Sivakumaran, N.; Krishnan, K.R.; Nayak, P.K.; Sahu, F.M.; Singha, S. Performance evaluation and economic analysis of modified solar dryer with thermal energy storage for drying of blood fruit (Haematocarpus validus). J. Food Process. Preserv. 2021, 45, e15653. [Google Scholar] [CrossRef]
- Feng, L.; Xu, Y.; Xiao, Y.; Song, J.; Li, D.; Zhang, Z.; Liu, C.; Liu, C.; Jiang, N.; Zhang, M.; et al. Effects of pre-drying treatments combined with explosion puffing drying on the physicochemical properties, antioxidant activities and flavor characteristics of apples. Food Chem. 2021, 338, 128015. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, B.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Identification of phenolic compounds in Australian grown dragon fruits by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Arab. J. Chem. 2021, 14, 103151. [Google Scholar] [CrossRef]
- Kondareddy, R.; Sivakumaran, N.; Radhakrishnan, K.; Nayak, P.K. Performance analysis of solar tunnel dryer with thermal storage and Photovoltaic system for drying star fruit. IOP Conf. Ser. Earth Environ. Sci. 2020, 463, 012138. [Google Scholar] [CrossRef]
- Dal-Bó, V.; Freire, J.T. Effects of lyophilization on colorimetric indices, phenolics content, and antioxidant activity of avocado (Persea americana) pulp. Food Control 2022, 132, 108526. [Google Scholar] [CrossRef]





| Parameters | HDS | HDP | FDS | FDP | VDS | VDP | RDS | RDP | Control |
|---|---|---|---|---|---|---|---|---|---|
| Drying time (min) | 1200 | 1200 | 1320 | 1320 | 1200 | 1200 | 900 | 840 | - |
| Ash (%) | 1.54 ± 0.02 a | 1.48 ± 0.03 b | 0.89 ± 0.01 ef | 0.84 ± 0.02 ef | 1.06 ± 0.03 g | 0.95 ± 0.04 d | 1.49 ± 0.01 cd | 1.34 ± 0.01 de | 0.65 ± 0.02 h |
| pH | 6.23 ± 0.01 g | 6.57 ± 0.02 c | 6.78 ± 0.01 f | 7.86 ± 0.01 b | 6.78 ± 0.01 f | 7.05 ± 0.01 d | 6.89 ± 0.02 e | 7.42 ± 0.01 da | 7.96 ± 0.025 h |
| TSS (°Brix) | 10.5 ± 0.2 bcde | 12 ± 0.2 ab | 9 ± 0.15 efgh | 10 ± 0.2 cdef | 9 ± 0.2 cd | 9.5 ± 0.2 bc | 10.5 ± 0.2 ef | 12 ± 0.2 bc | 13 ± 0.2 f |
| Crude fiber (%) | 0.86 ± 0.02 d | 0.91 ±0.22 cd | 0.72 ± 0.02 bc | 0.78 ±0.02 b | 0.82 ± 0.16 cd | 0.86 ± 0.02 bcd | 0.96 ± 0.02 bc | 0.98 ± 0.38 a | 1.14 ± 0.15 g |
| Texture | |||||||||
| Hardness (g) | 450.43 ± 5.62 a | 139.131 ± 3.21 b | 244.041 ± 4.32 ac | 23.60 ± 2.62 cd | 723.596 ± 8.62 c | 84.612 ± 3.41 d | 1089.33 ± 7.63 c | 101.959 ± 3.41 d | 18.952 ± 2.75 e |
| Fracturability (g) | 538.23 ± 16.32 a | ||||||||
| Adhesiveness (g.sec) | 0.18 ± 0.026 a | −1.791 ± 0.12 ba | −0.273 ± 0.076 ac | −5.191 ± 0.12 d | −2.544 ± 0.099 e | −0.667 ± 0.031 f | −5.220 ± 0.16 g | −1.213 ± 0.11 e | −0.089 ± 0.009 d |
| Springiness | 0.839 ± 0.12 d | 1.088 ± 0.92 c | 0.643 ± 0.098 g | 1.029 ± 0.45 cd | 0.714 ± 0.11 dg | 0.735 ±0.16 f | 0.982 ± 0.26 b | 1.118 ± 0.63 ab | 0.04 ± 0.0084 e |
| Cohesiveness | 0.622 ± 0.11 cd | 0.741 ± 0.099 ef | 0.488 ± 0.076 g | 0.842 ± 0.11 d | 0.647 ± 0.16 cd | 0.678 ± 0.22 d | 0.680 ± 0.10 b | 0.895 ± 0.16 a | 0.256 ± 0.043 f |
| Gumminess | 279.98 ± 8.36 c | 103.111 ± 3.45 cd | 119.048 ± 4.56 d | 1.988 ± 0.12 cd | 468.41 ± 5.625 f | 57.403 ± 1.23 bc | 741.246 ± 7.65 c | 91.302 ± 2.36 a | 109.86 ± 7.32 |
| Chewiness | 234.98 ± 4.56 a | 112.209 ± 3.69 d | 76.531 ± 1.63 b | 2.04 ± 0.765 d | 334.58 ± 7.89 g | 42.208 ± 6.54 d | 728.010 ± 11.12 a | 102.044 ± 3.69 b | 32.16 ± 1.25 g |
| Resilience | 0.250 ± 0.07 bc | 0.360 ± 0.083 a | 0.210 ± 0.062 ab | 0.26 ± 0.073 a | 0.19 ± 0.074 c | 0.213 ± 0.084 e | 0.288 ± 0.087 f | 0.328 ± 0.076 d | 0.162 ± 0.088 h |
| Color value | |||||||||
| L* | 16.28 ± 1.25 da | 23.46 ± 1.23 ce | 71.05 ± 4.32 c | 46.60 ± 2.52 a | 49.36 ± 2.65 b | 33.46 ± 1.56 ef | 42.486± 2.98 d | 24.70 ± 1.15 b | 66.23 ± 1.45 ac |
| a* | 7.91 ± 0.89 c | 1.74 ± 0.64 dg | −2.37 ± 0.88 bc | 4.69 ± 0.99 d | 9.41 ± 1.77 c | 7.1 ± 1.07 bc | 5.18 ± 0.88 a | 15.58 ± 1.01 c | 1.36 ± 0.25 g |
| b* | 6.08 ± 0.97 e | 0.627 ± 0.076 df | 20.32 ± 1.25 eg | 14.16 ± 1.06 b | 38.31 ± 2.69 a | 16.46 ± 1.06 d | 29.58 ± 1.99 c | 13.54 ± 1.01 c | 5.97 ± 0.95 ae |
| ∆ E | 60.09 ± 3.12 b | 59.95 ± 3.04 cd | 29.69 ± 1.19 d | 24.17 ± 1.97 a | 59.50 ± 2.76 ab | 59.41 ± 3.87 cd | 48.12 ± 2.99 cd | 46.64 ± 4.46 ac | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadhaneeya, H.; Kesavan, R.K.; Inbaraj, B.S.; Sharma, M.; Kamma, S.; Nayak, P.K.; Sridhar, K. Impact of Different Drying Methods on the Phenolic Composition, In Vitro Antioxidant Activity, and Quality Attributes of Dragon Fruit Slices and Pulp. Foods 2023, 12, 1387. https://doi.org/10.3390/foods12071387
Dadhaneeya H, Kesavan RK, Inbaraj BS, Sharma M, Kamma S, Nayak PK, Sridhar K. Impact of Different Drying Methods on the Phenolic Composition, In Vitro Antioxidant Activity, and Quality Attributes of Dragon Fruit Slices and Pulp. Foods. 2023; 12(7):1387. https://doi.org/10.3390/foods12071387
Chicago/Turabian StyleDadhaneeya, Harsh, Radha Krishnan Kesavan, Baskaran Stephen Inbaraj, Minaxi Sharma, Srinivasulu Kamma, Prakash Kumar Nayak, and Kandi Sridhar. 2023. "Impact of Different Drying Methods on the Phenolic Composition, In Vitro Antioxidant Activity, and Quality Attributes of Dragon Fruit Slices and Pulp" Foods 12, no. 7: 1387. https://doi.org/10.3390/foods12071387
APA StyleDadhaneeya, H., Kesavan, R. K., Inbaraj, B. S., Sharma, M., Kamma, S., Nayak, P. K., & Sridhar, K. (2023). Impact of Different Drying Methods on the Phenolic Composition, In Vitro Antioxidant Activity, and Quality Attributes of Dragon Fruit Slices and Pulp. Foods, 12(7), 1387. https://doi.org/10.3390/foods12071387