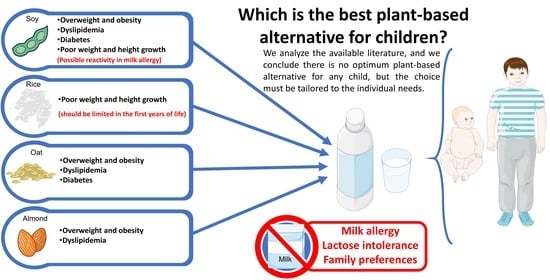
Plant-Based Milk Alternatives in Child Nutrition
Abstract
:1. Introduction
2. Main Nutritional Characteristics of Plant-Based Milk Alternatives Compared to Cow’s Milk
2.1. Plant-Based Formulas
- The allowed source of protein is cow’s milk, goat’s milk, isolated soy protein (ISP), and protein hydrolysates from “any suitable protein source and by different enzymatic or chemical means provided that the compositional criteria laid down by the Directive are met”. The minimum and maximum protein contents for IF and FOF in cow’s- and goat’s-derived formulas are 1.8 g/100 Kcal and 2.5 g/100 Kcal, respectively; for ISP 2.25 to 2.8 g/100 Kcal, respectively; and for protein hydrolyzates no minimum is set but the maximum is 2.8 g/100 Kcal. The Authors suggest a reference amino acidic pattern too [39]. As shown in Table 1, cow’s-milk protein content is 3.3 g/100 mL [40].
- Concerning the fat content, the EFSA panel proposes a fat content for IF and FOF that ranges between 40 and 55% of total energy, which is 4.4–6 g/100 Kcal of fat. The specific fat composition is detailed in particular for polyunsaturated fatty-acid requirements [39]. As a comparison, full-fat cow’s-milk fat content is around 3.2–3.7 g/100 mL [40]. In recent years, the European Union has made mandatory supplementation with docosahexaenoic acid for all formulas used in the first year of life [26].
- The carbohydrate content is calculated based on the residual energy after considering protein and fat composition. It can range between 9 g/100 Kcal and 14 g/100 Kcal for all kinds of formulas. Specific types of carbohydrates are detailed, including non-digestible carbohydrates [39]. As shown in Table 1, cow’s-milk carbohydrate content is 4.6 g/100 mL [40].
- The EFSA panel sets micronutrient composition for minerals and vitamins and discusses other ingredients such as choline, inositol, taurine, and probiotics, whose use can be planned in formulas. It is worth mentioning that higher amounts of iron are suggests for FOF compared to IF (minimum 0.6 mg/100 Kcal vs. minimum 0.3 mg/100 Kcal, respectively) when of animal origin and in protein hydrolyzates. ISP formulas should have a minimum iron content of 0.45 mg/100 Kcal for IF and 0.90 mg/100 Kcal for FOF [39]. In cow’s milk, iron is virtually absent [40].
- Finally, the EFSA panel does not consider it necessary to propose a specific composition of formulas used after 1 year of age, as “formula consumed during the first year of age can continue to be used by young children” [39]. The ESPGHAN position paper about young-child formulas (YCF) [41] agrees that follow-on formulas can be used after 1 year of age but calls for a “regulation of YCF to avoid inappropriate composition”.
2.2. Plant-Based Drinks
- Plant-based drinks vary in energy content depending on the source of the product and the possible presence of added sugar. Usually, almond-based drinks are the lowest in calories but can vary widely (e.g., 26–46 Kcal/100 mL [11], 25–74 Kcal/100 mL [42]), whereas rice-based drinks are the richest ones (e.g., 54–61 Kcal/100 mL [11], 47–68 Kcal/100 mL [42]). Soy-based drinks have ~45 Kcal/100 mL [11,42]. Full-fat cow’s milk has ~60 Kcal/100 mL [40].
- Plant-based drinks vary in protein amount, too, as shown in Table 2. The most similar to cow’s-milk content (i.e., 3.3 g/100 mL [40]) are soy-based drinks (3.3 g/100 mL [11], 3.1 g/100 mL [42]), whereas rice-based drinks have the lowest protein content (~0.2 g/100 mL [11] and 0.3 g/100 mL [42]). Protein quality is different as well. Even though we have not found in the literature specific data about the quality of protein in plant-based drinks, it is known that protein of animal origin is of better quality when compared to plant protein due to the different aminoacidic profile and the lower digestibility [43]. However, it is possible to improve the quality of the diet through the careful planning of a single meal and the overall diet [44,45].
- Regarding the fat content, rice-based drinks usually have the lowest values of ~1 g/100 mL [11,42]. Soy-based drinks have ~2 g/100 mL [11,42]. Full-fat cow’s milk has ~3.2–3.7 g/100 mL [40], whereas partially skimmed and fat-free cow’s milk have lower fat content. There are differences in the fatty-acid composition: a prevalence of saturated fatty acids is typical of cow’s milk (around 60%) and coconut-based drinks (around 90%) [10,36], whereas a prevalence of polyunsaturated fatty acids is reported for soy-based drinks [36]. Moreover, during the production, different oils (such as sunflower, rice) can be added to improve the organoleptic characteristics, making the lipid profile of the final product different from what could be inferred considering only the primary source.
- Rice-derived drinks usually are the richest in carbohydrates, with values slightly over 10 g/100 mL (12 g/100 mL [11], 11.5 g/100 mL [42], followed by oat-based drinks (7 to 8 g/100 mL) [11,42]. Soy-based drinks usually have a carbohydrate content ranging from 3 g/100 mL [11] to 4 g/100 mL [42]. Cow’s milk sits around 4.6 g/100 mL [40]. The main sugar in cow’s milk is lactose, whereas plant-based drinks are naturally devoid of lactose. However, sugars can be added to plant-based drinks, so the nutritional composition can vary widely according to sources and manufacturing.
- Whereas calcium is naturally present in cow’s milk (on average, 120 mg/100 mL), it can be added to plant-based drinks. Moreover, calcium bioavailability may differ depending on the type of fortification used (tricalcium phosphate, calcium carbonate, red alga Lithotamnium calcareum), even though the 2016 Academy of Nutrition and Dietetics position paper on vegetarian diets reports that calcium absorption “from most fortified plant milks is similar to that from cow’s milk, at approximately 30%” [46].
- Vitamin D, vitamin B2, and vitamin B12 can also be added to plant-based drinks, depending on the choice of the manufacturer. In the case of vitamin D, vitamin D3 can be of animal origin (from sheep lanolin), but in the last few years botanical sources of vitamin D3, Cladonia raingiferina (reindeer lichen), have become available [47].
3. The Role of Plant-Based Milk Alternatives in the Diets of Children 0–12 Months
3.1. Allergy to Cow’s-Milk Protein
3.2. Galactosemia and Lactose Intolerance
3.3. Preterm Infants
3.4. Choosing Plant-Based Milk Alternatives for Family Preferences
4. The Role of Plant-Based Milk Alternatives in the Diet of Children over 12 Months of Age
4.1. Allergy to Cow’s-Milk Proteins
4.2. Lactose Intolerance
4.3. Choosing Plant-Based Milk Alternatives for Family Preferences
Adequacy of Plant-Based Drinks Compared to Cow’s Milk
5. Advice Common to All Choices
5.1. Age
5.2. Quantity
6. Advice in Specific Situations
6.1. Overweight, Obesity, Dyslipidemia
6.2. Diabetes Mellitus
6.3. Poor Weight and Stature Growth
6.4. Allergy to Cow’s-Milk Proteins
6.5. Lactose Intolerance
6.6. Lacto-Ovo-Vegetarian and Vegan Diet or Family Preference for Plant-Based Milk Alternatives
- Perhaps with the exception of soy-based products, it may be advisable, especially in the second year of life, that a single source be not the only plant-based drink used to replace cow’s milk. It is suggested to offer plant-based drinks from different sources, alternating them during the week(s). This, among other factors, could add variety to the diet and encourage the acceptance of different foods in younger children.
- Due to the saturated-fat content of coconut-based drinks, these products are generally not recommended in children’s diets, except for occasional use.
- If a family chooses plant-based drinks from less common sources (flax, hemp, drinks with multiple components), it is essential to consider the nutritional information of the single product, always in the context of the individual’s overall diet.
6.6.1. In the Case of Using Plant-Based Drinks Derived from Soy
- It is suggested to choose drinks enriched with calcium (and possibly other minerals and vitamins) and free of added sugars.
- According to the CREA indications [74], for the 12–24-month age group, 150 mL of whole cow’s milk provides 90 Kcal, a value in line with the 80 Kcal per 200 mL of soy-based drink. One hundred fifty mL of whole cow’s milk provides 4.8 g of fats, a value slightly higher than that provided by 200 mL of soy-based drink (about 4 g) [40]. In this regard, beneath considering a possible additional source of fats in the meal, it should also be pointed out that the AAP allows the use of “low-fat” cow’s milk starting from the second year of life not only in a child with excess weight but also in a regularly growing child [70]. As for the protein intake, 200 mL of soy-based drink provides 5.6–7 g of proteins versus 5 g of proteins from 150 mL of cow’s milk [40]: This difference should not be a problem given the lower digestibility of plant protein.
- After the first 2 years of life, the official recommendation is that of switching from whole cow’s milk to partially or totally skimmed milk, with 200 mL per day [74]. About 100 Kcal are derived from 200 mL of 2% fat cow’s milk and 80 Kcal from 200 mL of soy-milk. We can consider the contributions of fat and protein to be roughly similar to that of cow’s milk: 200 mL of soy milk has about 4 g of fats, the same value as 200 mL of cow’s milk with 2% fat; regarding proteins, the intake from 200 mL of soy-based drink is 5.6–7 g, similar to 6.6–6.8 g per 200 mL of cow’s milk [40].
6.6.2. In the Case of Using Plant-Based Drinks Derived from Grains
- Limit the consumption of rice-based drinks in the first years of life (more or less for the first five years) due to the possible arsenic content.
- It is suggested to choose grain-based drinks enriched with calcium (and possibly other minerals and vitamins) and free of added sugars.
- Due to the carbohydrate content, sugars in particular, it may be advisable not to use rice-based drinks, and limit oat-based drinks, in a breakfast already rich in simple sugars (e.g., in a breakfast that includes bread and jam, biscuits, or cake prepared with added sugar). On the other hand, if we consider an older child or an adolescent who may exercise in the morning, grain-based drinks could be a choice to be considered.
- As for the 12–24-month range, 150 mL of whole cow’s milk provide 90 Kcal, 5 g of proteins, and 4.8 g of fats. Considering 200 mL of a grain-based drink, the contributions are similar in terms of calories (about 100 Kcal from 200 mL of rice- or oat-based drink) but different for proteins and lipids: For rice-based drink the same amount provides 0.6 g of proteins and 2 g of fats, and for oat-based drink 1.6 g of proteins and 5.4 of fats [40]. Therefore, it would be useful to provide another source of protein at breakfast if one of the above grain-based drinks is consumed, and another source of lipids in the case of rice-based drink.
- As for children 2 years of age and older, 200 mL of 2% fat cow’s milk provide 100 Kcal, 6.6–6.8 g of proteins, and 4 g of fats. As described above, the same amount of rice-based drink provides 100 Kcal, 0.6 g of proteins, and 2 g of fats; for 200 mL of oat-based drink the values are 100 Kcal, 1.6 g of proteins, and 5.4 g of fats [40]. Although the intakes of calories and lipids are similar, it would be useful to provide another source of protein in the case of grain-derived drinks.
6.6.3. In the Case of Using Plant-Based Drinks Derived from Almonds
- It is suggested to choose almond-based drinks enriched with calcium (and possibly other minerals and vitamins) and free of added sugars.
- Compared to the values of 150 mL of whole cow’s milk (90 Kcal, 5 g of proteins, and 4.8 g of fats), 200 mL of almond-based drink provide 30–40 Kcal, 1.2–1.4 g of proteins, and 2.4–3.2 g of fats [40]. It would therefore be advisable to include another source of protein and fat in the meal.
- Compared to 200 mL of semi-skimmed milk, the intake of 200 mL of almond-based drink remains lower in calories (100 Kcal vs. 30–40 Kcal, respectively) and proteins (6.6–6.8 g vs. 1.2–1.4 g) but similar in terms of fats (4 g vs. 2.4–3.2 g) [40]. Therefore, it would be useful to include another source of protein in the meal.
7. Limitations
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dougkas, A.; Barr, S.; Reddy, S.; Summerbell, C.D. A Critical Review of the Role of Milk and Other Dairy Products in the Development of Obesity in Children and Adolescents. Nutr. Res. Rev. 2019, 32, 106–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fructuoso, I.; Romão, B.; Han, H.; Raposo, A.; Ariza-Montes, A.; Araya-Castillo, L.; Zandonadi, R.P. An Overview on Nutritional Aspects of Plant-Based Beverages Used as Substitutes for Cow’s Milk. Nutrients 2021, 13, 2650. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and Health Attributes of Milk and Milk Imitations. Eur. J. Nutr. 2020, 59, 19–34. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Mulder, N.; De Greef, E.; Huysentruyt, K. Plant-Based Formulas and Liquid Feedings for Infants and Toddlers. Nutrients 2021, 13, 4026. [Google Scholar] [CrossRef]
- Vanga, S.K.; Raghavan, V. How Well Do Plant Based Alternatives Fare Nutritionally Compared to Cow’s Milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef]
- Vázquez-Frias, R.; Icaza-Chávez, M.E.; Ruiz-Castillo, M.A.; Amieva-Balmori, M.; Argüello-Arévalo, G.A.; Carmona-Sánchez, R.I.; Flores-Bello, M.V.; Hernández-Rosiles, V.; Hernández-Vez, G.; Medina-Vera, I.; et al. Technical Opinion of the Asociación Mexicana de Gastroenterología on Soy Plant-Based Beverages. Rev. Gastroenterol. México (Engl. Ed.) 2020, 85, 461–471. [Google Scholar] [CrossRef]
- European Union. Commission Delegated Regulation (UE) 2016/127. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02016R0127-20220401&qid=1680187465264 (accessed on 29 March 2023).
- European Union. Regulation (EU) No 609/2013 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02013R0609-20210428&qid=1680187465264 (accessed on 29 March 2023).
- Vandenplas, Y.; Brough, H.A.; Fiocchi, A.; Miqdady, M.; Munasir, Z.; Salvatore, S.; Thapar, N.; Venter, C.; Vieira, M.C.; Meyer, R. Current Guidelines and Future Strategies for the Management of Cow’s Milk Allergy. J. Asthma Allergy 2021, 14, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant Based Alternatives to Conventional Milk, Production, Potential and Health Concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3005–3023. [Google Scholar] [CrossRef]
- Angelino, D.; Rosi, A.; Vici, G.; Dello Russo, M.; Pellegrini, N.; Martini, D.; on behalf of the SINU Young Working Group. Nutritional Quality of Plant-Based Drinks Sold in Italy: The Food Labelling of Italian Products (FLIP) Study. Foods 2020, 9, 682. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-Dairy Plant-Based Milk Substitutes and Fermented Dairy-Type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Singhal, S.; Baker, R.D.; Baker, S.S. A Comparison of the Nutritional Value of Cow’s Milk and Nondairy Beverages. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Hughes, J.; Grafenauer, S. Got Mylk? The Emerging Role of Australian Plant-Based Milk Alternatives as A Cow’s Milk Substitute. Nutrients 2020, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Fresán, U. International Analysis of the Nutritional Content and a Review of Health Benefits of Non-Dairy Plant-Based Beverages. Nutrients 2021, 13, 842. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Kopf-Bolanz, K.A. Nutritional Implications of an Increasing Consumption of Non-Dairy Plant-Based Beverages Instead of Cow’s Milk in Switzerland. J. Adv. Dairy Res. 2017, 5, 1000197. [Google Scholar] [CrossRef]
- Berni-Canani, R.; Pezzella, V.; Amoroso, A.; Cozzolino, T.; Di Scala, C.; Passariello, A. Diagnosing and Treating Intolerance to Carbohydrates in Children. Nutrients 2016, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y. Lactose Intolerance. Asia Pac. J. Clin. Nutr. 2015, 24, S9–S13. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of Physicochemical and Glycaemic Properties of Commercial Plant-Based Milk Substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Clegg, M.E.; Tarrado Ribes, A.; Reynolds, R.; Kliem, K.; Stergiadis, S. A Comparative Assessment of the Nutritional Composition of Dairy and Plant-Based Dairy Alternatives Available for Sale in the UK and the Implications for Consumers’ Dietary Intakes. Food Res. Int. 2021, 148, 110586. [Google Scholar] [CrossRef]
- ESPGHAN Committee on Nutrition; Agostoni, C.; Axelsson, I.; Goule, O.; Koletzko, B.; Michaelsen, K.F.; Puntis, J.; Rieu, D.; Rigo, J.; Shamir, R.; et al. Soy Protein Infant Formulae and Follow-on Formulae: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s Milk Substitutes for Children: Nutritional Aspects of Milk from Different Mammalian Species, Special Formula and Plant-Based Beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, J.; Greer, F.; the Committee on Nutrition. Use of Soy Protein-Based Formulas in Infant Feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef] [Green Version]
- Commissione Europea, Regolamento Delegato (UE) 2016/127 REGOLAMENTO DELEGATO (UE) 2016/127 DELLA COMMISSIONE-del 25 Settembre 2015-Che Integra il Regolamento (UE) n. 609/ 2013 del Parlamento Europeo e del Consiglio per Quanto Riguarda le Prescrizioni Specifiche di Composizione e di Informazione per le Formule per Lattanti e le Formule di Proseguimento e per Quanto Riguarda le Prescrizioni Relative alle Informazioni Sull’alimentazione del Lattante e del Bambino Nella Prima Infanzia 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02016R0127-20220401&qid=1680187465264 (accessed on 30 March 2023).
- Dupont, C.; Bocquet, A.; Tomé, D.; Bernard, M.; Campeotto, F.; Dumond, P.; Essex, A.; Frelut, M.-L.; Guénard-Bilbault, L.; Lack, G.; et al. Hydrolyzed Rice Protein-Based Formulas, a Vegetal Alternative in Cow’s Milk Allergy. Nutrients 2020, 12, 2654. [Google Scholar] [CrossRef] [PubMed]
- Commissione Europea, REGOLAMENTO (UE) 2015/1006 REGOLAMENTO (UE) 2015/1006 DELLA COMMISSIONE-del 25 Giugno 2015-Recante Modifica del Regolamento (CE) n. 1881/2006 per Quanto Riguarda i Tenori Massimi di Arsenico Inorganico nei Prodotti Alimentari 2015. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=CELEX%3A32015R1006 (accessed on 30 March 2023).
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-Based Milk Substitutes: Bioactive Compounds, Conventional and Novel Processes, Bioavailability Studies, and Health Effects. J. Funct. Food 2020, 70, 103975. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Hoie, L.H.; Sjoholm, Å.; Guldstrand, M.; Zunft, H.-J.F.; Lüeder, W.; Graubaum, H.-J.; Gruenwald, J. Ultra Heat Treatment Destroys Cholesterol-Lowering Effect of Soy Protein. Int. J. Food Sci. Nutr. 2006, 57, 512–519. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Bosch-Fusté, J.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Changes of Isoflavones and Protein Quality in Soymilk Pasteurised by Ultra-High-Pressure Homogenisation throughout Storage. Food Chem. 2014, 162, 47–53. [Google Scholar] [CrossRef]
- Clark, B.E.; Pope, L.; Belarmino, E.H. Perspectives from Healthcare Professionals on the Nutritional Adequacy of Plant-Based Dairy Alternatives: Results of a Mixed Methods Inquiry. BMC Nutr. 2022, 8, 46. [Google Scholar] [CrossRef]
- Fifi, A.C.; Pagan, D.N.; Chakraborty, P.; Mahajan, N.; Axelrod, C.; Bayes, L.Y.; Katz, D.T. Physician and Parent Perceptions on Plant-Based Beverages as Substitutes for Cow’s Milk: A Single City Survey. J. Pediatr. Gastroenterol. Nutr. 2022, 75, e25–e29. [Google Scholar] [CrossRef]
- Adamczyk, D.; Jaworska, D.; Affeltowicz, D.; Maison, D. Plant-Based Dairy Alternatives: Consumers’ Perceptions, Motivations, and Barriers—Results from a Qualitative Study in Poland, Germany, and France. Nutrients 2022, 14, 2171. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Padilla, E.; Faber, I.; Petersen, I.L.; Vargas-Bello-Pérez, E. Perceptions toward Plant-Based Milk Alternatives among Young Adult Consumers and Non-Consumers in Denmark: An Exploratory Study. Foods 2023, 12, 385. [Google Scholar] [CrossRef]
- Moss, R.; Barker, S.; Falkeisen, A.; Gorman, M.; Knowles, S.; McSweeney, M.B. An Investigation into Consumer Perception and Attitudes towards Plant-Based Alternatives to Milk. Food Res. Int. 2022, 159, 111648. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Plant-Based Milk Alternatives (PBMA). Available online: https://www.fda.gov/food/food-labeling-nutrition/plant-based-milk-alternatives-pbma. (accessed on 29 March 2023).
- EFSA, European Food Safety Authority Scientific Opinion on the Essential Composition of Infant and Follow-on Formulae. EFS2 2014, 12, 3760. [CrossRef] [Green Version]
- USDA FoodData Central. Available online: https://fdc.nal.usda.gov (accessed on 28 December 2022).
- Hojsak, I.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hulst, J.; Indrio, F.; Lapillonne, A.; Mølgaard, C.; et al. Young Child Formula: A Position Paper by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Vitoria, I. The Nutritional Limitations of Plant-Based Beverages in Infancy and Childhood. Nutr. Hosp. 2017, 34, 1205–1214. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Messina, V.; Mangels, A.R. Considerations in Planning Vegan Diets: Children. J. Am. Diet. Assoc. 2001, 101, 661–669. [Google Scholar] [CrossRef]
- Mariotti. Gardner Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [Green Version]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Göring, H. Vitamin D in Nature: A Product of Synthesis and/or Degradation of Cell Membrane Components. Biochem. Moscow 2018, 83, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.; Toschi Vespasiani, G.; Ricci, G.; Miniaci, A.; di Palmo, E.; Pession, A. Cow’s Milk Protein Allergy as a Model of Food Allergies. Nutrients 2021, 13, 1525. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, N. Eosinophilic Gastrointestinal Disorders. Clin. Rev. Allergy Immunol. 2019, 57, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; World Allergy Organization (WAO) Special Committee on Food Allergy. World Allergy Organization (WAO) World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatr. Allergy Immunol. 2010, 21, 1–125. [Google Scholar] [CrossRef] [Green Version]
- Merritt, R.J.; Fleet, S.E.; Fifi, A.; Jump, C.; Schwartz, S.; Sentongo, T.; Duro, D.; Rudolph, J.; Turner, J. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper: Plant-Based Milks. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 276–281. [Google Scholar] [CrossRef]
- Haskovic, M.; Coelho, A.I.; Bierau, J.; Vanoevelen, J.M.; Steinbusch, L.K.M.; Zimmermann, L.J.I.; Villamor-Martinez, E.; Berry, G.T.; Rubio-Gozalbo, M.E. Pathophysiology and Targets for Treatment in Hereditary Galactosemia: A Systematic Review of Animal and Cellular Models. J. Inherit. Metab. Dis. 2020, 43, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Lak, R.; Yazdizadeh, B.; Davari, M.; Nouhi, M.; Kelishadi, R. Newborn Screening for Galactosaemia. Cochrane Database Syst. Rev. 2017, 12, CD012272. [Google Scholar] [CrossRef]
- Health Canada, Canadian Paediatric Society, Dietitians of Canada, and Breastfeeding Committee for Canada Nutrition for Healthy Term Infants: Recommendations from Six to 24 Months 2015. Available online: https://www.canada.ca/en/health-canada/services/canada-food-guide/resources/infant-feeding/nutrition-healthy-term-infants-recommendations-birth-six-months/6-24-months.html (accessed on 19 December 2022).
- Government of Australia, National Health and Medical Research Council Infant Feeding Guidelines Information for Health Workers 2012. Available online: https://www.nhmrc.gov.au/about-us/publications/infant-feeding-guidelines-information-health-workers#block-views-block-file-attachments-content-block-1 (accessed on 19 December 2022).
- Richter, M.; Boeing, H.; Grünewald-Funk, D.; Heseker, H.; Kroke, A.; Leschik-Bonnet, E.; Oberritter, H.; Strohm, D.; Watzl, B. for the German Nutrition Society (DGE) Vegan Diet. Position of the German Nutrition Society (DGE). Ernahr. Umsch. 2016, 63, 92–102. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Castrellon, P.G.; Rivas, R.; Gutiérrez, C.J.; Garcia, L.D.; Jimenez, J.E.; Anzo, A.; Hegar, B.; Alarcon, P. Safety of Soya-Based Infant Formulas in Children. Br. J. Nutr. 2014, 111, 1340–1360. [Google Scholar] [CrossRef]
- Baroni, L.; Goggi, S.; Battino, M. Planning Well-Balanced Vegetarian Diets in Infants, Children, and Adolescents: The VegPlate Junior. J. Acad. Nutr. Diet 2019, 119, 1067–1074. [Google Scholar] [CrossRef] [Green Version]
- Lemale, J.; Mas, E.; Jung, C.; Bellaiche, M.; Tounian, P. Vegan Diet in Children and Adolescents. Recommendations from the French-Speaking Pediatric Hepatology, Gastroenterology and Nutrition Group (GFHGNP). Arch. Pédiatrie 2019, 26, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Redecillas-Ferreiro, S.; Moráis-López, A.; Manuel Moreno-Villares, J. Position Paper on Vegetarian Diets in Infants and Children. Committee on Nutrition and Breastfeeding of the Spanish Paediatric Association. An. Pediatría (Engl. Ed.) 2020, 92, 306.e1–306.e6. [Google Scholar] [CrossRef]
- Ferrara, P.; Corsello, G.; Quattrocchi, E.; Dell’Aquila, L.; Ehrich, J.; Giardino, I.; Pettoello-Mantovani, M. Caring for Infants and Children Following Alternative Dietary Patterns. J. Pediatr. 2017, 187, 339–340.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, G.; Fadnes, L.; Meltzer, H.M.; Arnesen, E.K.; Henriksen, C. Follow-up of Pregnant Women, Breastfeeding Mothers and Infants on a Vegetarian or Vegan Diet. Tidsskr. Nor. Laegeforen. 2022, 142, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.; et al. Vegan Nutrition for Mothers and Children: Practical Tools for Healthcare Providers. Nutrients 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Academy of Pediatrics, Committee on Nutrition. Formula Feeding of Term Infants, Chapter. 4. In Pediatric Nutrition; American Academy of Pediatrics: Itasca, IL, USA, 2019; pp. 79–112. [Google Scholar]
- American Academy of Pediatrics, Committee on Nutrition. Nutritional Aspects of Vegetarian Diets, Chapter. 11. In Pediatric Nutrition; American Academy of Pediatrics: Itasca, IL, USA, 2019; pp. 293–319. [Google Scholar]
- Mangels, R.; Messina, V.; Messina, M. Vegetarian Diets in Infancy, Chapter. 13. In The Dietitian’s Guide to Vegetarian Diets: Issues and Applications; Jones & Bartlett Learning: Sudbury, MA, USA, 2021; pp. 273–290. [Google Scholar]
- Government of New Zealand, Ministry of Health. Healthy Eating Guidelines for New Zealand Babies and Toddlers (0–2 Years Old); Ministry of Health: Wellington, New Zealand, 2021; ISBN 978-1-991007-38-4. [Google Scholar]
- Lott, M.; Callahan, E.; Welker Duffy, E.; Story, M.; Daniels, S. Healthy Beverage Consumption in Early Childhood: Recommendations from Key National Health and Nutrition Organizations.Technical Scientific Report; Healthy Eating Research. 2019. Available online: http://healthyeatingresearch.org (accessed on 19 December 2022).
- Mangels, R.; Driggers, J. The Youngest Vegetarians: Vegetarian Infants and Toddlers. ICAN Infant Child Adolesc. Nutr. 2012, 4, 8–20. [Google Scholar] [CrossRef] [Green Version]
- American Academy of Pediatrics, Committee on Nutrition. Complementary Feeding, Chap. 6. In Pediatric Nutrition; American Academy of Pediatrics: Itasca, IL, USA, 2019; pp. 163–186. [Google Scholar]
- Vegan Society. A Nutrition Guide for Vegans under Five Years Old. Available online: https://www.vegansociety.com/sites/default/files/uploads/downloads/Under-fives%20PDF%202_0_0.pdf (accessed on 12 October 2022).
- European Commission. Report from the Commission to the European Parliament and the Council on Young Child Formulae; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- British Dietetic Association (BDA). Complementary Feeding (Weaning): Food Fact Sheet; British Dietetic Association: Birmingham, UK, 2020. [Google Scholar]
- CREA, Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria, Via della Navicella 2/4, 00184 Roma. Available online: https://www.crea.gov.it/web/alimenti-e-nutrizione/-/linee-guida-per-una-sana-alimentazione-2018 (accessed on 19 December 2022).
- Wright, N.S.; Smith, M. Guidelines Suggesting Children Avoid Plant-Based Milks: A Closer Examination. Matern. Child Health J. 2020, 24, 1189–1192. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Government of New Zealand, Ministry of Health. Food and Nutrition Guidelines for Healthy Children and Young People (Aged 2–18 Years): A Background Paper; Partial revision February 2015 (minor editorial changes); Ministry of Health: Wellington, New Zealand, 2015; ISBN 978-0-478-44482-7. [Google Scholar]
- Mangels, R.; Messina, V.; Messina, M. Toddlers, Preschoolers and School-Aged Children, Chapter. 14. In The Dietitian’s Guide to Vegetarian Diets: Issues and Applications; Jones & Bartlett Learning: Sudbury, MA, USA, 2021; pp. 291–311. [Google Scholar]
- Suthutvoravut, U.; Abiodun, P.O.; Chomtho, S.; Chongviriyaphan, N.; Cruchet, S.; Davies, P.S.W.; Fuchs, G.J.; Gopalan, S.; van Goudoever, J.B.; De La Rey Nel, E.; et al. Composition of Follow-Up Formula for Young Children Aged 12–36 Months: Recommendations of an International Expert Group Coordinated by the Nutrition Association of Thailand and the Early Nutrition Academy. Ann. Nutr. Metab. 2015, 67, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Governo Italiano, Ministero della Salute Corretta Alimentazione Ed Educazione Nutrizionale Nella Prima Infanzia F.A.Q 2016. Available online: https://www.salute.gov.it/portale/p5_1_1.jsp?lingua=italiano&faqArea=allattamento&id=201 (accessed on 19 December 2022).
- Verduci, E.; Di Profio, E.; Corsello, A.; Scatigno, L.; Fiore, G.; Bosetti, A.; Zuccotti, G.V. Which Milk during the Second Year of Life: A Personalized Choice for a Healthy Future? Nutrients 2021, 13, 3412. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on Nutrient Requirements and Dietary Intakes of Infants and Young Children in the European Union. EFS2 2013, 11, 3408. [CrossRef] [Green Version]
- Domellöf, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron Requirements of Infants and Toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and Its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics, AND, Vegetarian Nutrition Dietetic Practice Group. Vegetarian Infants. Available online: https://higherlogicdownload.s3.amazonaws.com/THEACADEMY/859dd171-3982-43db-8535-56c4fdc42b51/UploadedImages/VN/Documents/Resources/Vegetarian-Infants-Consumer.pdf (accessed on 12 October 2022).
- Baroni, L.; Goggi, S.; Battino, M. VegPlate: A Mediterranean-Based Food Guide for Italian Adult, Pregnant, and Lactating Vegetarians. J. Acad. Nutr. Diet. 2018, 118, 2235–2243. [Google Scholar] [CrossRef] [Green Version]
- Menal-Puey, S.; Martínez-Biarge, M.; Marques-Lopes, I. Developing a Food Exchange System for Meal Planning in Vegan Children and Adolescents. Nutrients 2018, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.; Shafiee, M.; Vatanparast, H. Trends in the Consumption of Conventional Dairy Milk and Plant-based Beverages and Their Contribution to Nutrient Intake among Canadians. J. Hum. Nutr. Diet. 2021, 34, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Rogero, M.M.; Fisberg, M.; Waitzberg, D. Health Impact of Childhood and Adolescent Soy Consumption. Nutr. Rev. 2017, 75, 500–515. [Google Scholar] [CrossRef] [Green Version]
- Eslami, O.; Shidfar, F. Soy Milk: A Functional Beverage with Hypocholesterolemic Effects? A Systematic Review of Randomized Controlled Trials. Complement. Ther. Med. 2019, 42, 82–88. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A Review of Health-Beneficial Properties of Oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef]
- Aly, E.; Sánchez-Moya, T.; Darwish, A.A.; Ros-Berruezo, G.; López-Nicolás, R. In Vitro Digestion Effect on CCK and GLP-1 Release and Antioxidant Capacity of Some Plant-based Milk Substitutes. J. Food Sci. 2022, 87, 1999–2008. [Google Scholar] [CrossRef]
- Fornari, E.; Brusati, M.; Maffeis, C. Nutritional Strategies for Childhood Obesity Prevention. Life 2021, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics, Committee on Nutrition. Dyslipidemias, Chapter. 32. In Pediatric Nutrition; American Academy of Pediatrics: Itasca, IL, USA, 2019; pp. 909–925. [Google Scholar]
- de Beer, H. Dairy Products and Physical Stature: A Systematic Review and Meta-Analysis of Controlled Trials. Econ. Hum. Biol. 2012, 10, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Morency, M.-E.; Birken, C.S.; Lebovic, G.; Chen, Y.; L’Abbé, M.; Lee, G.J.; Maguire, J.L. Association between Noncow Milk Beverage Consumption and Childhood Height. Am. J. Clin. Nutr. 2017, 106, 597–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catassi, C.; Agostoni, A.; Diamanti, A. L’alimentazione in Età Prescolare, Scolare e Nell’adolescenza, Chapter. 23. In Manuale SIGENP di Nutrizione Pediatrica; Il Pensiero Scientifico Editore: Rome, Italy, 2016; pp. 145–152. [Google Scholar]
- Guillod-Magnin, R.; Brüschweiler, B.J.; Aubert, R.; Haldimann, M. Arsenic Species in Rice and Rice-Based Products Consumed by Toddlers in Switzerland. Food Addit. Contam. Part A 2018, 35, 1164–1178. [Google Scholar] [CrossRef] [Green Version]
| Energy (Kcal/100 mL) | Protein (g/100 Kcal) | Lipids (g/100 Kcal) | Carbohydrates (g/100 Kcal) | |
|---|---|---|---|---|
| Animal-milk formula | 60–70 | 1.8–2.5 | 4.4–6 | 9–14 |
| ISP formula | 60–70 | 2.25–2.8 | 4.4–6 | 9–14 |
| HP formula | 60–70 | max. 2.8 | 4.4–6 | 9–14 |
| Full-fat cow’s milk | 60 | 3.3 | 3.3 | 4.6 |
| Energy (Kcal/100 mL) | Protein (g/100 mL) | Lipids (g/100 mL) | Carbohydrates (g/100 mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Soy | 44 * | 46.7 § | 3.3 * | 3.1 § | 2.0 * | 1.8 § | 3.0 * | 4.3 § |
| Rice | 57 * | 56.8 § | 0.2 * | 0.3 § | 1.0 * | 0.9 § | 12.0 * | 11.5 § |
| Almond | 38 * | 40.2 § | 0.8 * | 0.8 § | 2.3 * | 2.0 § | 3.0 * | 4.4 § |
| Oat | 47 * | 45.3 § | 0.6 * | 0.9 § | 1.2 * | 1.1 § | 7.9 * | 7.5 § |
| Full-fat cow’s milk | 60 | 3.3 | 3.3 | 4.6 | ||||
| Situation | <6 Months | ≥6 Months |
|---|---|---|
| Non-severe CMPA | EHF Rice | EHF Rice Soy * |
Severe CMPA, such as:
| AAF | AAF |
| CMPA, in the presence of symptoms not resolved by EHF | AAF Rice | AAF Rice Soy * |
| CMPA, in the presence of problems with EHFs | AAF Rice | AAF Rice Soy * |
| CMPA, in the presence of problems with AAFs | Rice | Rice Soy * |
| Situation | Choice |
|---|---|
| Obesity Overweight Dyslipidemia | - Soy - Almond - Oat - Spelt - Limit rice - Limit cow’s milk, prefer semi-skimmed milk - Formulas: 12–36-months |
| Types 1 and 2 diabetes mellitus | - Soy - Oat - Limit rice |
| Poor weight and height growth | - Cow’s milks - Soy - Rice * |
| CMPA | - Milk formulated for CMPA - Plant-based drinks * |
| Lactose intolerance | - Lactose-free cow’s milk - Plant-based drinks * |
| Family preference | - Plant-based drinks * |
| Age | Cow | Soy | Rice | Oat | Almond | Other |
|---|---|---|---|---|---|---|
| 12–24 months | 150 mL Whole Suitable as such | 200 mL Suitable as such * | 200 mL Provide protein and fat in the meal | 200 mL Provide protein in the meal | 200 mL Provide protein and fat in the meal | Evaluate on a case-by-case basis |
| >24 months | 200 mL Partially or totally skimmed Suitable as such | 200 mL Suitable as such | 200 mL Provide protein in the meal | 200 mL Provide protein in the meal | 200 mL Provide protein in the meal | Evaluate on a case-by-case basis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brusati, M.; Baroni, L.; Rizzo, G.; Giampieri, F.; Battino, M. Plant-Based Milk Alternatives in Child Nutrition. Foods 2023, 12, 1544. https://doi.org/10.3390/foods12071544
Brusati M, Baroni L, Rizzo G, Giampieri F, Battino M. Plant-Based Milk Alternatives in Child Nutrition. Foods. 2023; 12(7):1544. https://doi.org/10.3390/foods12071544
Chicago/Turabian StyleBrusati, Marco, Luciana Baroni, Gianluca Rizzo, Francesca Giampieri, and Maurizio Battino. 2023. "Plant-Based Milk Alternatives in Child Nutrition" Foods 12, no. 7: 1544. https://doi.org/10.3390/foods12071544
APA StyleBrusati, M., Baroni, L., Rizzo, G., Giampieri, F., & Battino, M. (2023). Plant-Based Milk Alternatives in Child Nutrition. Foods, 12(7), 1544. https://doi.org/10.3390/foods12071544