Author Contributions
Project administration, resources, funding acquisition, supervision, investigation, methodology, and visualisation, W.Y.K.; data curation, writing—original draft, and formal analysis, W.Y.K. and X.X.L.; writing—review and editing, W.Y.K., X.X.L., T.C.T., H.M., R.K. and B.R. All authors have read and agreed to the published version of the manuscript.
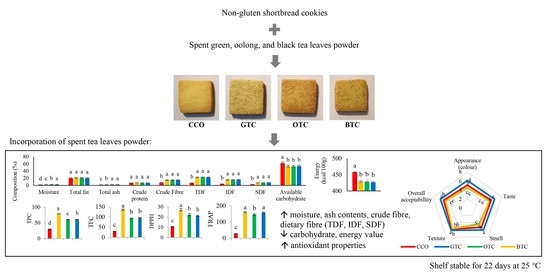
Figure 1.
Non-gluten shortbread cookies with various spent tea leaf powders: (a) control non-gluten shortbread cookies without spent tea leaves powder (CCO), (b) non-gluten shortbread cookies with spent green tea leaves powder (GTC), (c) non-gluten shortbread cookies with spent oolong tea leaves powder (OTC), and (d) non-gluten shortbread cookies with spent black tea leaves powder (BTC).
Figure 1.
Non-gluten shortbread cookies with various spent tea leaf powders: (a) control non-gluten shortbread cookies without spent tea leaves powder (CCO), (b) non-gluten shortbread cookies with spent green tea leaves powder (GTC), (c) non-gluten shortbread cookies with spent oolong tea leaves powder (OTC), and (d) non-gluten shortbread cookies with spent black tea leaves powder (BTC).
Figure 2.
The antioxidative properties (a) total phenolics content, (b) the total flavonoid content, (c) DPPH radical scavenging activity, and (d) ferric reducing antioxidant power (FRAP) of non-gluten shortbread cookies with various spent tea leaf powders. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a–d cookie samples). CCO = control; GTC = non-gluten shortbread cookie with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; and BTC = non-gluten shortbread cookies with spent black tea leaves powder.
Figure 2.
The antioxidative properties (a) total phenolics content, (b) the total flavonoid content, (c) DPPH radical scavenging activity, and (d) ferric reducing antioxidant power (FRAP) of non-gluten shortbread cookies with various spent tea leaf powders. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a–d cookie samples). CCO = control; GTC = non-gluten shortbread cookie with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; and BTC = non-gluten shortbread cookies with spent black tea leaves powder.
Figure 3.
Sensory properties of non-gluten shortbread cookies with various spent tea leaf powders. The mean ± SD (n 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a–c cookie samples). CCO = control; GTC = non-gluten shortbread cookie with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; BTC = non-gluten shortbread cookies with spent black tea leaves powder; 1 = extremely dislike; 2 = moderately dislike; 3 = slightly dislike; 4 = neither like nor dislike; 5 = slightly like; 6 = moderately like; and 7 = extremely like.
Figure 3.
Sensory properties of non-gluten shortbread cookies with various spent tea leaf powders. The mean ± SD (n 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a–c cookie samples). CCO = control; GTC = non-gluten shortbread cookie with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; BTC = non-gluten shortbread cookies with spent black tea leaves powder; 1 = extremely dislike; 2 = moderately dislike; 3 = slightly dislike; 4 = neither like nor dislike; 5 = slightly like; 6 = moderately like; and 7 = extremely like.
Figure 4.
The moisture content of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a–c cookie samples, A–D storage). CCO = control; GTC = non-gluten shortbread cookies with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; and BTC = non-gluten shortbread cookies with spent black tea leaves powder.
Figure 4.
The moisture content of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a–c cookie samples, A–D storage). CCO = control; GTC = non-gluten shortbread cookies with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; and BTC = non-gluten shortbread cookies with spent black tea leaves powder.
Figure 5.
Water activity (aw) of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a,b cookie samples, A–C storage). CCO = control; GTC = non-gluten shortbread cookies with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; and BTC = non-gluten shortbread cookies with spent black tea leaves powder.
Figure 5.
Water activity (aw) of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a,b cookie samples, A–C storage). CCO = control; GTC = non-gluten shortbread cookies with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; and BTC = non-gluten shortbread cookies with spent black tea leaves powder.
Figure 6.
The hardness of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a,b cookie samples, A–D storage). CCO = control; GTC = non-gluten shortbread cookies with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; and BTC = non-gluten shortbread cookies with spent black tea leaves powder.
Figure 6.
The hardness of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a,b cookie samples, A–D storage). CCO = control; GTC = non-gluten shortbread cookies with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; and BTC = non-gluten shortbread cookies with spent black tea leaves powder.
Figure 7.
The colour (a) lightness, (b) redness, and (c) yellowness of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a,b cookie samples, A storage). CCO = control; GTC = non-gluten shortbread cookies with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; BTC = non-gluten shortbread cookies with spent black tea leaves powder; and L* lightness, a* redness, and b* yellowness.
Figure 7.
The colour (a) lightness, (b) redness, and (c) yellowness of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a,b cookie samples, A storage). CCO = control; GTC = non-gluten shortbread cookies with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; BTC = non-gluten shortbread cookies with spent black tea leaves powder; and L* lightness, a* redness, and b* yellowness.
Figure 8.
The sensory properties (a) appearance, (b) taste, (c) smell, (d) texture, and (e) overall acceptability of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a–c cookie samples, A,B storage). CCO = control; GTC = non-gluten shortbread cookie with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; BTC = non-gluten shortbread cookies with spent black tea leaves powder; 1 = extremely dislike; 2 = moderately dislike; 3 = slightly dislike; 4 = neither like nor dislike; 5 = slightly like; 6 = moderately like; and 7 = extremely like.
Figure 8.
The sensory properties (a) appearance, (b) taste, (c) smell, (d) texture, and (e) overall acceptability of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C. The mean ± SD (n = 3). Different letters indicate significant differences between the values (Duncan’s multiple range test, p < 0.05, a–c cookie samples, A,B storage). CCO = control; GTC = non-gluten shortbread cookie with spent green tea leaves powder; OTC = non-gluten shortbread cookies with spent oolong tea leaves powder; BTC = non-gluten shortbread cookies with spent black tea leaves powder; 1 = extremely dislike; 2 = moderately dislike; 3 = slightly dislike; 4 = neither like nor dislike; 5 = slightly like; 6 = moderately like; and 7 = extremely like.
Table 1.
Formulation of non-gluten shortbread cookies with various spent tea leaf powders.
Table 1.
Formulation of non-gluten shortbread cookies with various spent tea leaf powders.
| Ingredients (% w/w) | Cookies |
|---|
| CCO | GTC | OTC | BTC |
|---|
| Rice flour | 32 | 24 | 24 | 24 |
| Maize flour | 15 | 15 | 15 | 15 |
| Spent tea leaves powder | 0 | 8 | 8 | 8 |
| Unsalted butter | 2.5 | 2.5 | 2.5 | 2.5 |
| Sugar | 24 | 24 | 24 | 24 |
| Egg yolk | 11.5 | 11.5 | 11.5 | 11.5 |
| Almond powder | 15 | 15 | 15 | 15 |
Table 2.
Proximate composition, energy value, and tannin content of non-gluten shortbread cookies with various spent tea leaf powders.
Table 2.
Proximate composition, energy value, and tannin content of non-gluten shortbread cookies with various spent tea leaf powders.
| Component | Cookies |
|---|
| CCO | GTC | OTC | BTC |
|---|
| Moisture (%) | 1.62 ± 0.24 c | 2.18 ± 0.03 b | 2.35 ± 0.01 a | 2.19 ± 0.01 b |
| Crude fat (%) | 20.17 ± 0.03 a | 20.60 ± 0.55 a | 20.80 ± 0.38 a | 20.39 ± 0.46 a |
| Total ash (%) | 1.43 ± 0.04 c | 2.04 ± 0.04 a | 1.91 ± 0.06 b | 1.96 ± 0.04 b |
| Crude protein (%) | 6.86 ± 0.10 a | 6.88 ± 0.04 a | 6.89 ± 0.10 a | 6.84 ± 0.05 a |
| Crude fibre (%) | 7.70 ± 0.04 b | 14.47 ± 0.45 a | 14.89 ± 0.09 a | 14.75 ± 0.06 a |
| Total dietary fibre (%) | 6.82 ± 1.35 b | 22.38 ± 1.07 a | 22.59 ± 1.28 a | 22.48 ± 1.16 a |
| Insoluble dietary fibre (%) | 4.15 ± 0.62 b | 15.32 ± 0.78 a | 15.44 ± 0.99 a | 15.83 ± 1.63 a |
| Soluble dietary fibre (%) | 2.67 ± 0.79 b | 7.06 ± 0.38 a | 7.15 ± 0.35 a | 7.66 ± 0.50 a |
| Total carbohydrate (%) | 62.21 ± 0.68 a | 53.83 ± 0.80 b | 53.16 ± 0.48 b | 53.87 ± 0.48 b |
| Energy (kcal/100 g) | 457.84 ± 0.71 a | 428.21 ± 2.68 b | 427.36 ± 1.80 b | 426.37 ± 2.44 b |
| Tannin (mg/100 g) | 0.28 ± 0.31 d | 2.33 ± 0.27 c | 4.22 ± 0.79 b | 6.10 ± 0.26 a |
Table 3.
The density and pH of the dough of non-gluten shortbread cookies with various spent tea leaf powders.
Table 3.
The density and pH of the dough of non-gluten shortbread cookies with various spent tea leaf powders.
| Cookies | Density (g/mL) | pH |
|---|
| CCO | 1.27 ± 0.05 a | 7.75 ± 0.03 a |
| GTC | 1.08 ± 0.08 a | 7.15 ± 0.01 c |
| OTC | 1.07 ± 0.06 a | 7.16 ± 0.01 c |
| BTC | 1.05 ± 0.09 a | 7.38 ± 0.02 b |
Table 4.
Spread factor, loss rate, and hardness of non-gluten shortbread cookies with various spent tea leaf powders.
Table 4.
Spread factor, loss rate, and hardness of non-gluten shortbread cookies with various spent tea leaf powders.
| Cookies | Texture |
|---|
| Spread Factor | Loss Rate (%) | Hardness (g) |
|---|
| CCO | 4.58 ± 0.02 c | 13.76 ± 0.05 b | 3242.32 ± 384.18 a |
| GTC | 4.98 ± 0.01 b | 12.01 ± 0.04 c | 3391.29 ± 359.72 a |
| OTC | 5.36 ± 0.02 a | 13.65 ± 0.07 b | 3398.64 ± 142.54 a |
| BTC | 5.01 ± 0.03 b | 15.08 ± 0.04 a | 3374.28 ± 531.18 a |
Table 5.
Colours of non-gluten shortbread cookies with various spent tea leaves powder.
Table 5.
Colours of non-gluten shortbread cookies with various spent tea leaves powder.
| Cookies | Colour Parameters |
|---|
| L* | a* | b* |
|---|
| CCO | 77.78 ± 0.65 a | 6.35 ± 0.48 a | 40.71 ± 0.92 a |
| GTC | 60.43 ± 1.12 b | 2.81 ± 0.15 b | 27.08 ± 1.36 b |
| OTC | 59.03 ± 0.98 b | 2.65 ± 0.29 c | 26.98 ± 0.22 b |
| BTC | 58.41 ± 1.03 b | 2.89 ± 0.21 b | 26.84 ± 0.74 b |
Table 6.
The water activity of non-gluten shortbread cookies with various spent tea leaf powders.
Table 6.
The water activity of non-gluten shortbread cookies with various spent tea leaf powders.
| Cookies | Water Activity |
|---|
| CCO | 0.15 ± 0.01 b |
| GTC | 0.23 ± 0.02 a |
| OTC | 0.24 ± 0.02 a |
| BTC | 0.22 ± 0.02 a |
Table 7.
Microbiological quality of non-gluten shortbread cookies with various spent tea leaf powders.
Table 7.
Microbiological quality of non-gluten shortbread cookies with various spent tea leaf powders.
| Microbial Load (CFU/g) | Reference Value (Maximum Level) * | Cookies |
|---|
| CCO | GTC | OTC | BTC |
|---|
| Total aerobic mesophilic | 1 × 104 CFU/g | <10 | <10 | <10 | <10 |
| Yeasts and moulds | 1 × 102 CFU/g | <10 | <10 | <10 | <10 |
| Staphylococcus spp. | 1 × 10 CFU/g | <10 | <10 | <10 | <10 |
| Salmonella spp. | Absent in 25 g | Absent | Absent | Absent | Absent |
| Total coliform | 1 × 10 CFU/g | <10 | <10 | <10 | <10 |
| Escherichia coli | Absent in 10 g | Absent | Absent | Absent | Absent |
| Bacillus cereus | 1 × 10 CFU/g | <10 | <10 | <10 | <10 |
Table 8.
The microbiological quality of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C.
Table 8.
The microbiological quality of non-gluten shortbread cookies with various spent tea leaf powders during different storage periods (day 1, 8, 15, and 22) at 25 °C.
| Microbial Load (CFU/g) | Reference Value (Maximum Level) * | Days | Cookies |
|---|
| CCO | GTC | OTC | BTC |
|---|
| Total aerobic mesophilic | 1 × 104 CFU/g | 1 | <10 | <10 | <10 | <10 |
| 8 | <10 | <10 | <10 | <10 |
| 15 | <10 | <10 | <10 | <10 |
| 22 | 2.41 × 102 | 2.68 × 102 | 2.23 × 102 | 2.55 × 102 |
| Yeasts and moulds | 1 × 102 CFU/g | 1 | <10 | <10 | <10 | <10 |
| 8 | <10 | <10 | <10 | <10 |
| 15 | <10 | <10 | <10 | <10 |
| 22 | <10 | <10 | <10 | <10 |
| Staphylococcus spp. | 1 × 10 CFU/g | 1 | <10 | <10 | <10 | <10 |
| 8 | <10 | <10 | <10 | <10 |
| 15 | <10 | <10 | <10 | <10 |
| 22 | <10 | <10 | <10 | <10 |
| Salmonella | Absent in 25 g | 1 | Absent | Absent | Absent | Absent |
| 8 | Absent | Absent | Absent | Absent |
| 15 | Absent | Absent | Absent | Absent |
| 22 | Absent | Absent | Absent | Absent |
| Total coliform | 1 × 10 CFU/g | 1 | <10 | <10 | <10 | <10 |
| 8 | <10 | <10 | <10 | <10 |
| 15 | <10 | <10 | <10 | <10 |
| 22 | <10 | <10 | <10 | <10 |
| Escherichia coli | Absent in 10 g | 1 | Absent | Absent | Absent | Absent |
| 8 | Absent | Absent | Absent | Absent |
| 15 | Absent | Absent | Absent | Absent |
| 22 | Absent | Absent | Absent | Absent |
| Bacillus cereus | 1 × 10 CFU/g | 1 | <10 | <10 | <10 | <10 |
| 8 | <10 | <10 | <10 | <10 |
| 15 | <10 | <10 | <10 | <10 |
| 22 | <10 | <10 | <10 | <10 |