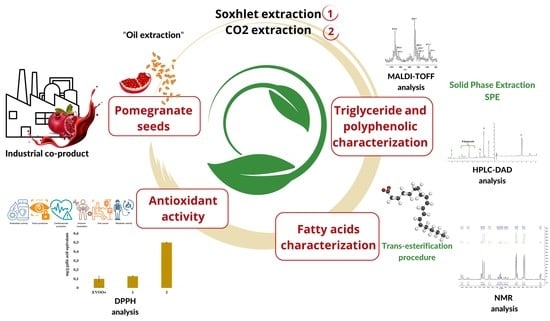
In-Depth Chemical Characterization of Punica granatum L. Seed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Soxhlet Extraction with n-Hexane
2.3. CO2-EtOH Supercritical Extraction
2.4. Atmospheric Pressure Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric (AP-MALDI-MS) Analysis
2.5. Transesterification Procedure
H and 13C-NMR Analysis
2.6. Polyphenols Separation by Solid-Phase Extraction
2.7. HPLC-DAD Analysis
2.8. DPPH (2,2-diphenyl-1-picryl-hydrazyl) Assay
2.9. Statistical Analysis
3. Results
3.1. Extraction Procedures
3.2. AP-MALDI-MS Analysis
3.3. NMR Analysis
3.4. Solid Phase Extraction, HPLC-DAD and DPPH Analysis
3.5. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Puneeth, H.R.; Chandra, S.S.P. A review on potential therapeutic properties of Pomegranate (Punica granatum L.). Plant Sci. Today 2020, 7, 9–16. [Google Scholar] [CrossRef]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Fourati, M.; Smaoui, S.; Hlima, H.B.; Elhadef, K.; Braïek, O.B.; Ennouri, K.; Mtibaa, A.C.; Mellouli, L. Bioactive compounds and pharmacological potential of pomegranate (Punica granatum) seeds-a review. Plant Foods Hum. Nutr. 2020, 75, 477–486. [Google Scholar] [CrossRef]
- Tarantino, A.; Difonzo, G.; Disciglio, G.; Frabboni, L.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Fresh pomegranate juices from cultivars and local ecotypes grown in southeastern Italy: Comparison of physicochemical properties, antioxidant activity and bioactive compounds. J. Sci. Food Agric. 2022, 102, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Pizzolongo, F.; Romano, R.; Di Matteo, M. Study of pomological traits and physico-chemical quality of pomegranate (Punica granatum L.) genotypes grown in Italy. Eur. Food Res. Technol. 2018, 244, 1427–1438. [Google Scholar] [CrossRef]
- Arlotta, C.; Toscano, V.; Genovese, C.; Calderaro, P.; Puglia, G.D.; Raccuia, S.A. Nutraceutical Content and Genetic Diversity Share a Common Pattern in New Pomegranate Genotypes. Molecules 2022, 27, 389. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Pitonzo, R.; Novara, M.E.; Bongiorno, D.; Indelicato, S.; Gentile, C.; Avellone, G.; Bognanni, R.; Scandurra, S.; Melilli, M.G. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC–Orbitrap-MS approach. J. Sci. Food Agric. 2019, 99, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Boggia, R.; Donno, D.; Parodi, B.; Beccaro, G.; Baldassari, S.; Signorello, M.G.; Catena, S.; Alfei, S.; Zunin, P. From pomegranate marcs to a potential bioactive ingredient: A recycling proposal for pomegranate-squeezed marcs. Eur. Food Res. Technol. 2020, 246, 273–285. [Google Scholar] [CrossRef]
- Bahmani, M.; Shokri, S.; Nosrati Akhtar, Z.; Abbaszadeh, S.; Manouchehri, A. The effect of pomegranate seed oil on human health, especially epidemiology of polycystic ovary syndrome; a systematic review. JBRA Assist. Reprod. 2022, 26, 631–636. [Google Scholar] [CrossRef]
- Hennessy, A.A.; Ross, R.P.; Devery, R.; Stanton, C. The health promoting properties of the conjugated isomers of α-linolenic acid. Lipids 2011, 46, 105–119. [Google Scholar] [CrossRef]
- Fontes, A.L.; Pimentel, L.L.; Simões, C.D.; Gomes, A.M.; Rodríguez-Alcalá, L.M. Evidences and perspectives in the utilization of CLNA isomers as bioactive compounds in foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 2611–2622. [Google Scholar] [CrossRef] [PubMed]
- Boroushaki, M.T.; Mollazadeh, H.; Afshari, A.R. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Rev. Res. 2016, 7, 430. [Google Scholar]
- Bañares, C.; Chabni, A.; de Donlebún, B.P.; Reglero, G.; Torres, C.F. Chemical characterization of pomegranate and alfalfa seed oils obtained by a two-step sequential extraction procedure of expeller and supercritical CO2 technologies. J. Food Compos. Anal. 2023, 115, 105040. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Khoddami, A.; Man, Y.B.C.; Roberts, T.H. Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur. J. Lipid Sci. Technol. 2014, 116, 553–562. [Google Scholar] [CrossRef]
- Cakaloglu, B.; Ozyurt, V.H.; Ötleş, S. Cold press in oil extraction. A review. Ukr. Food J. 2018, 7, 640–654. [Google Scholar] [CrossRef]
- Eikani, M.H.; Golmohammad, F.; Rowshanzamir, S. Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). J. Food Eng. 2007, 80, 735–740. [Google Scholar] [CrossRef]
- Gumus, Z.P.; Argon, Z.U.; Celenk, V.U. Cold pressed pomegranate (Punica granatum) seed oil. In Cold Pressed Oils; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 597–609. [Google Scholar]
- Liu, G.; Xu, X.; Hao, Q.; Gao, Y. Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. LWT-Food Sci. Technol. 2009, 42, 1491–1495. [Google Scholar] [CrossRef]
- Ahangari, B.; Sargolzaei, J. Extraction of pomegranate seed oil using subcritical propane and supercritical carbon dioxide. Theor. Found. Chem. Eng. 2012, 46, 258–265. [Google Scholar] [CrossRef]
- Natolino, A.; Da Porto, C. Supercritical carbon dioxide extraction of pomegranate (Punica granatum L.) seed oil: Kinetic modelling and solubility evaluation. J. Supercrit. Fluids 2019, 151, 30–39. [Google Scholar] [CrossRef]
- Kaufman, M.; Wiesman, Z. Pomegranate oil analysis with emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J. Agric. Food Chem. 2007, 55, 10405–10413. [Google Scholar] [CrossRef]
- Suzuki, R.; Noguchi, R.; Ota, T.; Abe, M.; Miyashita, K.; Kawada, T. Cytotoxic effect of conjugated trienoic fatty acids on mouse tumor and human monocytic leukemia cells. Lipids 2001, 36, 477–482. [Google Scholar] [CrossRef]
- Cairone, F.; Petralito, S.; Scipione, L.; Cesa, S. Study on Extra Virgin Olive Oil: Quality Evaluation by Anti-Radical Activity, Color Analysis, and Polyphenolic HPLC-DAD Analysis. Foods 2021, 10, 1808. [Google Scholar] [CrossRef]
- Cairone, F.; Garzoli, S.; Menghini, L.; Simonetti, G.; Casadei, M.A.; Di Muzio, L.; Cesa, S. Valorization of kiwi peels: Fractionation, bioactives analyses and hypotheses on complete peels recycle. Foods 2022, 11, 589. [Google Scholar] [CrossRef]
- Buchgraber, M.; Ulberth, F.; Emons, H.; Anklam, E. Triacylglycerol profiling by using chromatographic techniques. Eur. J. Lipid Sci. Technol. 2004, 106, 621–648. [Google Scholar] [CrossRef]
- Almoselhy, R.I.; Allam, M.H.; El-Kalyoubi, M.H.; El-Sharkawy, A.A. 1H NMR spectral analysis as a new aspect to evaluate the stability of some edible oils. Ann. Agric. Sci. 2014, 59, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Gaydou, E.M.; Miralles, J.; Rasoazanakolona, V. Analysis of conjugated octadecatrienoic acids inmomordica balsamina seed oil by GLC and13C NMR spectroscopy. J. Am. Oil Chem. Soc. 1987, 64, 997–1000. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, H.L.; Chen, J.N.; Chen, Z.Y.; Yang, L. Identification and characterization of conjugated linolenic acid isomers by Ag+-HPLC and NMR. J. Agric. Food Chem. 2006, 54, 9004–9009. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, L.; Gao, H.L.; Chen, J.N.; Chen, Z.Y.; Ren, Q.S. Re-characterization of three conjugated linolenic acid isomers by GC–MS and NMR. Chem. Phys. Lipids 2007, 145, 128–133. [Google Scholar] [CrossRef]
- Sassano, G.; Sanderson, P.; Franx, J.; Groot, P.; van Straalen, J.; Bassaganya-Riera, J. Analysis of pomegranate seed oil for the presence of jacaric acid. J. Sci. Food Agric. 2009, 89, 1046–1052. [Google Scholar] [CrossRef]
- Verardo, V.; Garcia-Salas, P.; Baldi, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Pomegranate seeds as a source of nutraceutical oil naturally rich in bioactive lipids. Food Res. Int. 2014, 65, 445–452. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 2017, 221, 1883–1894. [Google Scholar] [CrossRef]
- He, L.; Xu, H.; Liu, X.; He, W.; Yuan, F.; Hou, Z.; Gao, Y. Identification of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant capacities by HPLC–ABTS+ assay. Food Res. Int. 2011, 44, 1161–1167. [Google Scholar] [CrossRef]
- Abbasi, H.; Rezaei, K.; Rashidi, L. Extraction of essential oils from the seeds of pomegranate using organic solvents and supercritical CO2. J. Am. Oil Chem. Soc. 2008, 85, 83–89. [Google Scholar] [CrossRef]
- Liu, G.; Xu, X.; Gong, Y.; He, L.; Gao, Y. Effects of supercritical CO2 extraction parameters on chemical composition and free radical-scavenging activity of pomegranate (Punica granatum L.) seed oil. Food Bioprod. Process. 2012, 90, 573–578. [Google Scholar] [CrossRef]





| Compound | 1b | 2b |
|---|---|---|
| Coumaric acid | 7.14 ± 0.22 | 3.61 ± 0.02 |
| Carvacrol | 20.50 ± 0.47 | 136.77 ± 1.33 |
| Timol | 108.63 ± 5.88 | 471.52 ± 2.60 |
| Total polyphenols 1 | 43.49 ± 1.52 | 99.57 ± 2.98 |
| Flavonols 2 | - 3 | 16.37 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cairone, F.; Salvitti, C.; Iazzetti, A.; Fabrizi, G.; Troiani, A.; Pepi, F.; Cesa, S. In-Depth Chemical Characterization of Punica granatum L. Seed Oil. Foods 2023, 12, 1592. https://doi.org/10.3390/foods12081592
Cairone F, Salvitti C, Iazzetti A, Fabrizi G, Troiani A, Pepi F, Cesa S. In-Depth Chemical Characterization of Punica granatum L. Seed Oil. Foods. 2023; 12(8):1592. https://doi.org/10.3390/foods12081592
Chicago/Turabian StyleCairone, Francesco, Chiara Salvitti, Antonia Iazzetti, Giancarlo Fabrizi, Anna Troiani, Federico Pepi, and Stefania Cesa. 2023. "In-Depth Chemical Characterization of Punica granatum L. Seed Oil" Foods 12, no. 8: 1592. https://doi.org/10.3390/foods12081592
APA StyleCairone, F., Salvitti, C., Iazzetti, A., Fabrizi, G., Troiani, A., Pepi, F., & Cesa, S. (2023). In-Depth Chemical Characterization of Punica granatum L. Seed Oil. Foods, 12(8), 1592. https://doi.org/10.3390/foods12081592