Effects of Steam Treatment Time and Drying Temperature on Properties of Sweet Basil’s Antioxidants, Aroma Compounds, Color, and Tissue Structure
Abstract
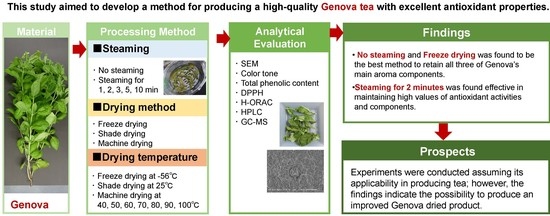
:1. Introduction
2. Materials and Methods
2.1. Materials for Experiments
2.2. Materials for Genova Tea
2.3. Genova Tea Manufacturing Method
2.4. SEM
2.5. Color
2.6. Sample Extraction
2.7. TPC and DPPH Radical Scavenging Assay and H-ORAC Assay
2.8. HPLC Analyses
2.9. Gas Chromatography–Mass Spectrometry
2.10. Statistical Analysis
3. Results and Discussion
3.1. TPC and Antioxidant Properties according to Plant Part
3.2. SEM
3.3. Color
3.4. TPC and Antioxidant Activities
3.5. Rosmarinic and Chicoric Acid Content
3.6. Aroma Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiltunen, R.; Holm, Y. Basil: The Genus Ocimum; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Ali, J.A.; Ovidiu, J.I.; Maria, D.E. Study on the cultivation of ‘Genovese’ and ‘Aromat de Buzău’ basil varieties in the NFT system. J. Hortic. For. Biotechnol. 2021, 25, 27–31. [Google Scholar]
- Mueen, A.C.; Syeda, B.N.; Asifa, S.; Maimoona, A.; Muhammad, A.S. Biological and Pharmacological Properties of the Sweet Basil (Ocimum basilicum). Br. J. Pharm. Res. 2015, 115, 330–339. [Google Scholar]
- Yamasaki, K.; Nakano, M.; Kawahata, T.; Mori, H.; Otake, T.; Ueda, N.; Oishi, I.; Inami, R.; Yamane, M.; Nakamura, M. Anti-HIV-1 activity of herbs in Labiatae. Biol. Pharm. Bull. 1998, 21, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Nakayama, S.; Kawazoe, K.; Takaishi, Y. Antiaggregant effects on human platelets of culinary herbs. Phytother. R. 1998, 12, 603–605. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics Composition and Antioxidant Activity of Sweet Basil (Ocimum basilicum L.). J. Agr. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Zunin, P.; Salvadeo, P.; Boggia, R.; Lanteri, S. Study of different kinds of “Pesto Genovese” by the analysis of their volatile fraction and chemometric methods. Food Chem. 2009, 114, 306–309. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Tajima, K.; Toi, N.; Sugimura, Y. Characteristic Components Found in the Essential Oil of Ocimum basilicum L. Flavour. Fragr. J. 1997, 12, 195–200. [Google Scholar] [CrossRef]
- Srivastava, S.; Cahill, D.M.; Conlan, X.A.; Adholeya, A. A novel in vitro whole plant system for analysis of polyphenolics and their antioxidant potential in cultivars of Ocimum Basilicum. J. Agr. Food Chem. 2014, 62, 10064–10075. [Google Scholar] [CrossRef]
- Liu, J.; Wan, Y.; Zhao, Z.; Chen, H. Determination of the content of rosmarinic acid by HPLC and analytical comparison of volatile constituents by GC-MS in different parts of Perilla frutescens (L.) Britt. Chem. Cent. J. 2013, 7, 61. [Google Scholar] [CrossRef]
- Arceusz, A.; Wesolowski, M. Quality consistency evaluation of Melissa officinalis L. commercial herbs by HPLC fingerprint and quantitation of selected phenolic acids. J. Pharm. Biomed. Anal. 2013, 83, 215–220. [Google Scholar] [CrossRef]
- De Masi, L.; Siviero, P.; Esposito, C.; Castaldo, D.; Siano, F.; Laratta, B. Assessment of agronomic, chemical and genetic variability in common basil (Ocimum basilicum L.). Eur. Food Res. Technol. 2006, 223, 273–281. [Google Scholar] [CrossRef]
- Abramovic, H.; Abram, V.; Cuk, A.; Ceh, B.; Smole Mozina, S.; Vidmar, M.; Pavlovic, M.; Poklar Ulrih, N. Antioxidative and antibacterial properties of organically grown thyme (Thymus sp.) and basil (Ocimum basilicum L.). Turk. J. Agric. 2018, 42, 185–194. [Google Scholar] [CrossRef]
- Sharma, R.; Bhatia, S.; Kaur, P. Effect of drying methods on biochemical quality of basil leaf. Agric. Res. J. 2018, 5, 331–335. [Google Scholar] [CrossRef]
- Boggia, R.; Leardi, R.; Zunin, P.; Bottino, A.; Capannelli, G. Dehydration of pdo genovese basil leaves (Ocimum basilicum maximum L. cv genovese gigante) by direct osmosis. J. Food Process. Preserv. 2013, 37, 621–629. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Kanou, M.; Ikeura, H.; Makino, M.; Oowatari, Y.; Tsuchiya, I. Effect of different tea manufacturing methods on the antioxidant activity, functional components, and aroma compounds of Ocimum gratissimum. LWT 2022, 169, 114058. [Google Scholar] [CrossRef]
- Watanabe, J.; Oki, T.; Takebayashi, J.; Yamasaki, K.; Takano-Ishikawa, Y.; Hino, A.; Yasui, A. Method validation by interlaboratory studies of improved hydrophilic oxygen radical absorbance capacity methods for the determination of antioxidant capacities of antioxidant solutions and food extracts. Anal. Sci. 2012, 28, 159–165. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Takahashi, T.; Nagata, Y. Production of persimmon and mandarin peel pastes and their uses in food. Food Sci. Nutr. 2021, 9, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Farneti, B.; Cristescu, S.M.; Costa, G.; Harren, F.J.M.; Woltering, E.J. Rapid tomato volatile profiling by using proton-transfer reaction mass spectrometry (PTR-MS). J. Food Sci. 2012, 77, C551–C559. [Google Scholar] [CrossRef] [PubMed]
- Schuurink, R.; Tissier, A. Glandular trichomes: Micro-organs with model status? New. Phytol. 2020, 225, 2251–2266. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, D.; Bonner, L.; Johnson, C.B. UV-B is required for normal development of oil glands in Ocimum basilicum L. (Sweet Basil). Ann. Bot. 2002, 90, 453–460. [Google Scholar] [CrossRef] [PubMed]
- de Santana, A.C.M.; Pereira, G.S.; Boaventura, C.M.; Uetenabaro, A.P.T.; Costa, L.C.d.B.; de Oliveira, R.A. Rupture of glandular trichomes in Ocimum gratissimum leaves influences the content of essential oil during the drying method. Rev. Bras. Farmacogn. 2014, 24, 524–530. [Google Scholar] [CrossRef]
- Amin, S.F.M.; Muhammad, K.; Karim, R.; Yusof, Y.A.; Mamat, H.; Sintang, M.D. A Short Review on the Stability of Chlorophylls and Metallo-Chlorophyll Complexes in Fruits and Vegetables. Trans. Sci. Technol. 2021, 8, 419–424. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Niemeyer, E.D. Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). J. Agr. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-H.; Wu, C.-S.; Wang, M.; Xu, B.-N.; Du, L.-J. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agr. Food Chem. 2012, 60, 9642–9648. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of traditional and novel drying techniques and its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Ünal, M.Ü.; Şener, A. Determination of some biochemical properties of polyphenol oxidase from Emir grape (Vitis vinifera L. cv. Emir). J. Sci. Food Agric. 2006, 86, 2374–2379. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Orphanides, A.; Goulas, V.; Gekas, V. Drying technologies: Vehicle to high-quality herbs. Food Eng. Rev. 2016, 8, 164–180. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Slimmon, T.; McAuley, C.Y.; Kott, L.S. Heat stress reduces the accumulation of rosmarinic acid and the total antioxidant capacity in spearmint (Mentha spicata L). J. Sci. Food Agric. 2005, 85, 2429–2436. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Tousch, D.; Lajoix, A.-D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Afolayan, A.J.; Muchenje, V. Phytochemical constituents and antioxidant activity of sweet basil (Ocimum basilicum L.) essential oil on ground beef from boran and nguni cattle. Int. J. Food Sci. 2019, 2019, 2628747. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.; Kumar, G.; Gandass, N.; Kajal; Verma, A.; Rajarammohan, S.; Rai, N.; Gautam, V. Antimicrobial Potential of Essential Oils from Aromatic Plant Ocimum sp.; A Comparative Biochemical Profiling and In-Silico Analysis. Agronomy 2022, 12, 627. [Google Scholar] [CrossRef]
- Xing, Y.; Lei, H.; Wang, J.; Wang, Y.; Wang, J.; Xu, H. Effects of different drying methods on the total phenolic, rosmarinic acid and essential oil of purple perilla leaves. J. Essent. Oil-Bear. Plants 2017, 20, 1594–1606. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Müller, J. Changes of essential oil content and composition during convective drying of lemon balm (Melissa officinalis L.). Ind. Crops Prod. 2014, 52, 118–124. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurunaga, Y.; Kanou, M. Effects of Steam Treatment Time and Drying Temperature on Properties of Sweet Basil’s Antioxidants, Aroma Compounds, Color, and Tissue Structure. Foods 2023, 12, 1663. https://doi.org/10.3390/foods12081663
Tsurunaga Y, Kanou M. Effects of Steam Treatment Time and Drying Temperature on Properties of Sweet Basil’s Antioxidants, Aroma Compounds, Color, and Tissue Structure. Foods. 2023; 12(8):1663. https://doi.org/10.3390/foods12081663
Chicago/Turabian StyleTsurunaga, Yoko, and Mina Kanou. 2023. "Effects of Steam Treatment Time and Drying Temperature on Properties of Sweet Basil’s Antioxidants, Aroma Compounds, Color, and Tissue Structure" Foods 12, no. 8: 1663. https://doi.org/10.3390/foods12081663
APA StyleTsurunaga, Y., & Kanou, M. (2023). Effects of Steam Treatment Time and Drying Temperature on Properties of Sweet Basil’s Antioxidants, Aroma Compounds, Color, and Tissue Structure. Foods, 12(8), 1663. https://doi.org/10.3390/foods12081663