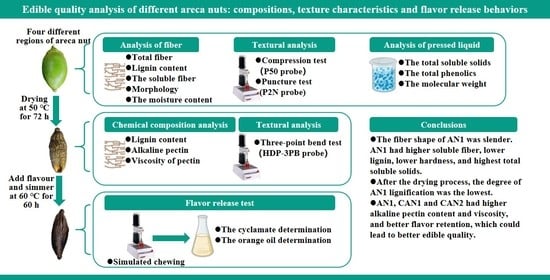
Edible Quality Analysis of Different Areca Nuts: Compositions, Texture Characteristics and Flavor Release Behaviors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of Fiber from the Areca Nut Samples
2.2.1. Total Fiber
2.2.2. Lignin Content
2.2.3. The Soluble Fiber
2.2.4. Alkaline Pectin
2.2.5. Morphological Characterization and the Moisture Content of the Areca Nut Fiber
2.3. Textural Analysis
2.3.1. Compression Test of Fresh Areca Nuts
2.3.2. Puncture Test of Fresh Areca Nuts
2.3.3. Fiber Hardness Test of Dried Areca Nuts
2.4. Analysis of Pressed Liquid from Areca Nuts
2.4.1. The Total Soluble Solids
2.4.2. The Total Phenolics
2.4.3. The Molecular Weight
2.5. Flavor Release Test
2.6. Statistical Analyses
3. Results
3.1. Visual Appearance of Areca Nuts
3.2. Analysis of Fiber from Areca Nuts
3.3. Composition of Fresh Areca Nuts
3.4. Textural Analysis of Fresh Areca Nuts
3.5. MW Distribution
3.6. Fiber Composition of Dried Areca Nuts
3.7. Textural Analysis of Dried Areca Nut Fiber
3.8. Alkaline Pectin
3.9. Flavor Release of Dried Areca Nuts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulino, Y.C.; Wilkens, L.R.; Sotto, P.P.; Franke, A.A.; Kawamoto, C.T.; Chennaux, J.S.; Mendez, A.J.; Tenorio, L.F.; Badowski, G.; Pokhrel, P.; et al. Rationale and design of a randomized, controlled, superiority trial on areca nut/betel quid cessation: The Betel Nut Intervention Trial (BENIT). Contemp. Clin. Trials Commun. 2020, 17, 100544. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Tulsyan, S.; Thakur, N.; Sharma, V.; Sinha, D.N.; Mehrotra, R. Chemistry, metabolism and pharmacology of carcinogenic alkaloids present in areca nut and factors affecting their concentration. Regul. Toxicol. Pharmacol. 2020, 110, 104548. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Malik, A.; Chowdhury, S.; Chauhan, S. Sociocultural aspects of areca nut use. Addict. Biol. 2002, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pandya, S.; Chaudhary, A.K.; Singh, M.; Singh, M.; Mehrotra, R. Correlation of histopathological diagnosis with habits and clinical findings in oral submucous fibrosis. Head Neck Oncol. 2009, 1, 10. [Google Scholar] [CrossRef]
- Yi, S.; Zou, L.; Li, Z.; Sakao, K.; Wang, Y.; Hou, D.-X. In Vitro Antioxidant Activity of Areca Nut Polyphenol Extracts on RAW264.7 Cells. Foods 2022, 11, 3607. [Google Scholar] [CrossRef]
- Jain, V.; Garg, A.; Parascandola, M.; Chaturvedi, P.; Khariwala, S.S.; Stepanov, I. Analysis of Alkaloids in Areca Nut-Containing Products by Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 1977–1983. [Google Scholar] [CrossRef]
- Dalisay, F.; Buente, W.; Benitez, C.; Herzog, T.A.; Pokhrel, P. Adolescent betel nut use in Guam: Beliefs, attitudes and social norms. Addict. Res. Theory 2019, 27, 394–404. [Google Scholar] [CrossRef]
- Chen, P.-H.; Tsai, C.-C.; Lin, Y.-C.; Ko, Y.-C.; Yang, Y.-H.; Shieh, T.-Y.; Ho, P.-S.; Li, C.-M.; Ko, A.M.-S.; Chen, C.-H. Ingredients Contribute to Variation in Production of Reactive Oxygen Species by Areca Quid. J. Toxicol. Environ. Health Part A 2006, 69, 1055–1069. [Google Scholar] [CrossRef]
- Ji, W.-T.; Lee, C.-I.; Chen, J.Y.-F.; Cheng, Y.-P.; Yang, S.-R.; Chen, J.-H.; Chen, H.-R. Areca Nut Extract Induces Pyknotic Necrosis in Serum-Starved Oral Cells via Increasing Reactive Oxygen Species and Inhibiting GSK3β: An Implication for Cytopathic Effects in Betel Quid Chewers. PLoS ONE 2013, 8, e63295. [Google Scholar] [CrossRef]
- Johnson, N.; Warnakulasuriya, S.; Gupta, P.; Dimba, E.; Chindia, M.; Otoh, E.; Sankaranarayanan, R.; Califano, J.; Kowalski, L.P. Global Oral Health Inequalities in Incidence and Outcomes for Oral Cancer. Adv. Dent. Res. 2011, 23, 237–246. [Google Scholar] [CrossRef]
- Li, W.-C.; Lee, P.-L.; Chou, I.-C.; Chang, W.-J.; Lin, S.-C.; Chang, K.-W. Molecular and cellular cues of diet-associated oral carcinogenesis-with an emphasis on areca-nut-induced oral cancer development. J. Oral Pathol. Med. 2014, 44, 167–177. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chem. 2020, 342, 128352. [Google Scholar] [CrossRef]
- Wu, M.; Liu, P.; Wang, S.; Zhong, C.; Zhao, X. Ultrasonic Microwave-Assisted Micelle Combined with Fungal Pretreatment of Eucommia ulmoides Leaves Significantly Improved the Extraction Efficiency of Total Flavonoids and Gutta-Percha. Foods 2021, 10, 2399. [Google Scholar] [CrossRef]
- Zheng, Z.; Deng, W.; Li, Y.; Song, H.; Chen, S. Extraction, physiological function and application of soluble dietary fiber from edible fungi: A review. Food Sci. Technol. 2022, 42, e35422. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Zhang, F.; Yang, X.; Ni, L.; Zhang, W.; Liu, Z.; Zhang, Y. Improving viscosity and gelling properties of leaf pectin by comparing five pectin extraction methods using green tea leaf as a model material. Food Hydrocoll. 2019, 98, 105246. [Google Scholar] [CrossRef]
- Ghoshal, G.; Negi, P. Isolation of pectin from kinnow peels and its characterization. Food Bioprod. Process. 2020, 124, 342–353. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, H.; Zhao, H.; Ren, H.; Zhai, H. Study on Dissociation and Chemical Structural Characteristics of Areca Nut Husk. Molecules 2023, 28, 1513. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, W.; Liu, Y.; Song, Y.; Wu, L.; Liu, C.; Wang, T. Water Status and Predictive Models of Moisture Content during Drying of Soybean Dregs Based on LF-NMR. Molecules 2022, 27, 4421. [Google Scholar] [CrossRef]
- An, X.; Li, Z.; Zude-Sasse, M.; Tchuenbou-Magaia, F.; Yang, Y. Characterization of textural failure mechanics of strawberry fruit. J. Food Eng. 2020, 282, 110016. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, N.; Xiang, C.; Li, Y.; Jiang, X.; Zeng, M.; Xu, H.; Wang, H.; Wu, H.; Yu, X.; et al. Three-Dimensional Printing Properties of Polysaccharide Hydrocolloids–Unrinsed Sturgeon Surimi Complex Hydrogels. Foods 2022, 11, 2947. [Google Scholar] [CrossRef]
- Wetchakama, S.; Prachayawarakorn, S.; Soponronnarit, S. Change of mechanical properties related to starch gelatinization and moisture content of rice kernel during fluidized bed drying. Dry. Technol. 2018, 37, 1173–1183. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.; Pei, J.; Meng, H.; Wang, H.; Li, Z. Evaluation of hybrid pressure-driven and osmotically-driven membrane process for non-thermal production of apple juice concentrate. Innov. Food Sci. Emerg. Technol. 2021, 75, 102895. [Google Scholar] [CrossRef]
- Hao, W.; Wang, S.-F.; Zhao, J.; Li, S.-P. Effects of extraction methods on immunology activity and chemical profiles of Lycium barbarum polysaccharides. J. Pharm. Biomed. Anal. 2020, 185, 113219. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, C.; Zhang, S.; Liu, X.; Luo, J.; Gao, H.; Zhang, H.; Wei, H. Simulated oral processing of cooked rice using texture analyzer equipped with multiple extrusion cell probe (TA/MEC). LWT 2020, 138, 110731. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, I.K.; Kim, H.; Lee, S. Artificial saliva-induced structural breakdown of rice flour gels under simulated chewing conditions. Food Sci. Biotechnol. 2018, 28, 387–393. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Li, B.; Xu, X.-J.; Ren, H.-B.; Yin, J.-Y.; Zhu, H.; Zhang, Y.-H. FTIR spectroscopy coupled with machine learning approaches as a rapid tool for identification and quantification of artificial sweeteners. Food Chem. 2020, 303, 125404. [Google Scholar] [CrossRef]
- Minhee, S.; Jeongmo, Y.; Sukhyeong, C.; Jinwon, L.; Han, B.O. GC–MS Method for the Quantitative Analysis of Limonene from Genetically Engineered Saccharomyces cerevisiae. Bull. Korean Chem. Soc. 2018, 39, 1368–1372. [Google Scholar]
- Guo, Y.; Pan, Y.; Zhang, Z.; Zhang, W. Study on the browning mechanism of betel nut (Betel catechu L.) Kernel. Food Sci. Nutr. 2020, 8, 1818–1827. [Google Scholar] [CrossRef]
- Shi, R.; Pang, C.; Wu, X.; Zhao, X.; Chen, F.; Zhang, W.; Sun, C.; Fu, S.; Hu, M.; Zhang, J.; et al. Genetic Dissection and Germplasm Selection of the Low Crude Fiber Component in Brassica napus L. Shoots. Foods 2023, 12, 403. [Google Scholar] [CrossRef]
- Nucci, D.; Santangelo, O.E.; Provenzano, S.; Fatigoni, C.; Nardi, M.; Ferrara, P.; Gianfredi, V. Dietary Fiber Intake and Risk of Pancreatic Cancer: Systematic Review and Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 11556. [Google Scholar] [CrossRef]
- Hu, L.; Yang, C.; Zhang, L.; Feng, J.; Xi, W. Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef]
- Ofoedu, C.E.; Ofoedu, E.O.; Chacha, J.S.; Owuamanam, C.I.; Efekalam, I.S.; Awuchi, C.G. Comparative Evaluation of Physicochemical, Antioxidant, and Sensory Properties of Red Wine as Markers of Its Quality and Authenticity. Int. J. Food Sci. 2022, 2022, 8368992. [Google Scholar] [CrossRef]
- Alafeef, A.K.; Ariffin, F.; Zulkurnain, M. Organic Selenium as Antioxidant Additive in Mitigating Acrylamide in Coffee Beans Roasted via Conventional and Superheated Steam. Foods 2020, 9, 1197. [Google Scholar] [CrossRef]
- Qin, W.; Sun, L.; Miao, M.; Zhang, G. Plant-sourced intrinsic dietary fiber: Physical structure and health function. Trends Food Sci. Technol. 2021, 118, 341–355. [Google Scholar] [CrossRef]
- Botinestean, C.; Keenan, D.F.; Kerry, J.P.; Hamill, R.M. The effect of thermal treatments including sous-vide, blast freezing and their combinations on beef tenderness of M. semitendinosus steaks targeted at elderly consumers. LWT 2016, 74, 154–159. [Google Scholar] [CrossRef]
- Peng, Z.; Jin, Y. Purification, identification, and characterization of an endo-1,4-β-xylanase from wheat malt. Molecules 2020, 25, 1572. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Villamiel, M.; Moreno, R.; Moreno, F.J. Structural and Rheological Properties of Pectins Extracted from Industrial Sugar Beet By-Products. Molecules 2019, 24, 392. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, J.; Wei, C.; Ye, X.; Shi, J.; Chen, S. Pectin from Citrus Canning Wastewater as Potential Fat Replacer in Ice Cream. Molecules 2018, 23, 925. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Yu, Y.; Huang, X. Effect of ultrasonic treatment on rheological and emulsifying properties of sugar beet pectin. Food Sci. Nutr. 2020, 8, 4266–4275. [Google Scholar] [CrossRef]
- Chen, J. Food oral processing—A review. Food Hydrocoll. 2007, 23, 1–25. [Google Scholar] [CrossRef]






| Areca Nuts | Length (mm) | Width (mm) | Length/Width |
|---|---|---|---|
| AN1 | 41.0 ± 3.1a | 24.3 ± 1.1a | 1.69 |
| AN2 | 42.1 ± 3.9a | 25.9 ± 3.5a | 1.63 |
| AN3 | 43.6 ± 4.1a | 25.3 ± 3.3a | 1.80 |
| AN4 | 42.2 ± 3.1a | 25.7 ± 3.4a | 1.64 |
| TSS (%) | Soluble Fiber (%) | Total Polyphenol (mg GAE/mL) | Lignin (%) | Total Fiber (%) | Moisture Content (%) | |
|---|---|---|---|---|---|---|
| AN1 | 4.94 ± 0.12a | 3.00 ± 0.24a | 2.76 ± 0.03b | 24.81 ± 1.80b | 26.07 ± 0.27a | 78.26 ± 0.42a |
| AN2 | 4.73 ± 0.18ab | 2.92 ± 0.25a | 3.27 ± 0.02a | 25.77 ± 1.74a | 26.71 ± 0.52a | 78.16 ± 1.27a |
| AN3 | 4.68 ± 0.15b | 2.77 ± 0.28a | 3.28 ± 0.05a | 28.31 ± 1.86a | 26.75 ± 0.62a | 77.32 ± 1.52a |
| AN4 | 4.72 ± 0.10ab | 2.86 ± 0.23a | 3.31 ± 0.03a | 26.01 ± 1.92a | 26.16 ± 0.52a | 77.74 ± 2.07a |
| Hardness (Puncture Test)/g | Hardness (Compression Test)/g | Chewiness (Compression Test)/g | Springiness (Compression Test) | |
|---|---|---|---|---|
| AN1 | 345.96 ± 141.45b | 23,775.91 ± 4502.57ab | 2223.06 ± 686.69b | 0.3038 ± 0.0253b |
| AN2 | 468.58 ± 158.65a | 25,892.62 ± 4653.75a | 3555.12 ± 698.38a | 0.3128 ± 0.0368ab |
| AN3 | 508.50 ± 163.71a | 28,270.76 ± 4777.90a | 3703.98 ± 767.44a | 0.3419 ± 0.0173a |
| AN4 | 498.74 ± 150.39a | 26,266.14 ± 4654.20a | 3595.29 ± 759.15a | 0.3269 ± 0.0294ab |
| Soluble Fiber/% | Lignin/% | |
|---|---|---|
| AN1 | 1.94 ± 0.09a | 26.07 ± 0.27c |
| AN2 | 1.62 ± 0.07b | 27.25 ± 0.47b |
| AN3 | 1.53 ± 0.06b | 28.71 ± 0.52a |
| AN4 | 1.58 ± 0.05b | 27.57 ± 0.50b |
| CAN1 | 1.36 ± 0.03c | 23.56 ± 0.33d |
| CAN2 | 1.31 ± 0.10c | 24.65 ± 0.76d |
| Hardness/g | Chewiness/g | Springiness | |
|---|---|---|---|
| AN1 | 9396.36 ± 2184.87c | 1422.44 ± 413.30a | 0.3006 ± 0.0364a |
| AN2 | 10,452.56 ± 4251.33bc | 1598.52 ± 455.38a | 0.3119 ± 0.0382a |
| AN3 | 12,554.55 ± 3952.30a | 1648.65 ± 513.32a | 0.3165 ± 0.0389a |
| AN4 | 11,855.95 ± 3125.41ab | 1552.34 ± 486.27a | 0.3163 ± 0.0375a |
| CAN1 | 6069.00 ± 2537.69d | 1025.61 ± 305.80b | 0.2157 ± 0.0242b |
| CAN2 | 6064.66 ± 1626.23d | 1035.29 ± 351.64b | 0.2189 ± 0.0218b |
| Content/g | Viscosity/Pa·s | |
|---|---|---|
| AN1 | 4.66 ± 0.12a | 0.0315 ± 0.0023a |
| AN2 | 4.15 ± 0.10bc | 0.0205 ± 0.0018b |
| AN3 | 4.06 ± 0.10c | 0.0215 ± 0.0021b |
| AN4 | 4.23 ± 0.15b | 0.0203 ± 0.0027b |
| CAN1 | 4.57 ± 0.05a | 0.0316 ± 0.0023a |
| CAN2 | 4.55 ± 0.06a | 0.0317 ± 0.0023a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Chen, H.; Chen, W.; Chen, W.; Zhong, Q.; Zhang, M.; Pei, J. Edible Quality Analysis of Different Areca Nuts: Compositions, Texture Characteristics and Flavor Release Behaviors. Foods 2023, 12, 1749. https://doi.org/10.3390/foods12091749
Yang B, Chen H, Chen W, Chen W, Zhong Q, Zhang M, Pei J. Edible Quality Analysis of Different Areca Nuts: Compositions, Texture Characteristics and Flavor Release Behaviors. Foods. 2023; 12(9):1749. https://doi.org/10.3390/foods12091749
Chicago/Turabian StyleYang, Bowen, Haiming Chen, Weijun Chen, Wenxue Chen, Qiuping Zhong, Ming Zhang, and Jianfei Pei. 2023. "Edible Quality Analysis of Different Areca Nuts: Compositions, Texture Characteristics and Flavor Release Behaviors" Foods 12, no. 9: 1749. https://doi.org/10.3390/foods12091749
APA StyleYang, B., Chen, H., Chen, W., Chen, W., Zhong, Q., Zhang, M., & Pei, J. (2023). Edible Quality Analysis of Different Areca Nuts: Compositions, Texture Characteristics and Flavor Release Behaviors. Foods, 12(9), 1749. https://doi.org/10.3390/foods12091749