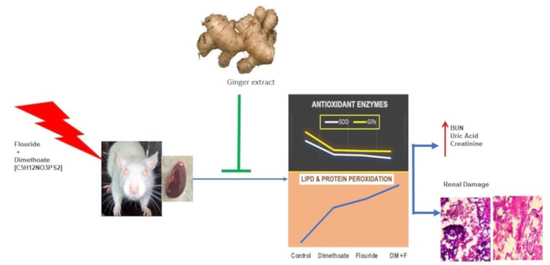
Protective Effect of Quercetin and Ginger (Zingiber officinale) Extract against Dimethoate Potentiated Fluoride-Induced Nephrotoxicity in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Zingiber officinale (ZO) Roscoe Extract
2.2. Instrumentation and Chromatographical Analysis
2.3. Experimental Design
2.4. Sample Collection and Analysis
2.5. Fluoride Estimation
2.6. Determination of Antioxidant Biomarkers in Renal Tissue
2.7. Histopathology
2.8. Statistical Analysis
3. Results
3.1. Chromatographical Analysis of the ZO Rhizome Extract
3.2. Fluoride Levels in the Plasma and Renal Tissues
3.3. Plasma and Renal Biomarkers
3.4. Effect on Plasma GSH and NO
3.5. Antioxidant Biomarkers in Renal Tissue
3.6. Histopathological Alterations in Renal Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Scoy, A.; Pennell, A.; Zhang, X. Environmental Fate and Toxicology of Dimethoate. Rev. Environ. Contam. Toxicol. 2016, 237, 53–70. [Google Scholar] [PubMed]
- Tuduri, L.; Harner, T.; Blanchard, P.; Li, Y.F.; Poissant, L.; Waite, D.T.; Murphy, C.; Belzer, W. A Review of Currently Used Pesticides (CUPs) in Canadian Air and Precipitation: Part 1: Lindane and Endosulfans. Atmos. Environ. 2006, 40, 1563–1578. [Google Scholar] [CrossRef]
- He, J.; Zhou, L.; Yao, Q.; Liu, B.; Xu, H.; Huang, J. Greenhouse and Field-Based Studies on the Distribution of Dimethoate in Cotton and Its Effect on Tetranychus Urticae by Drip Irrigation. Pest Manag. Sci. 2018, 74, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ben Amara, I.; Karray, A.; Hakim, A.; Ben Ali, Y.; Troudi, A.; Soudani, N.; Boudawara, T.; Zeghal, K.M.; Zeghal, N. Dimethoate Induces Kidney Dysfunction, Disrupts Membrane-Bound ATPases and Confers Cytotoxicity through DNA Damage. Protective Effects of Vitamin e and Selenium. Biol. Trace Elem. Res. 2013, 156, 230–242. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, P.K.; Sood, S.; Singh, M.; Verma, D. Impact of Chronic Sodium Fluoride Toxicity on Antioxidant Capacity, Biochemical Parameters, and Histomorphology in Cardiac, Hepatic, and Renal Tissues of Wistar Rats. Biol. Trace Elem. Res. 2023, 201, 229–241. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, P.K.; Sood, S.; Singh, R.; Gupta, A.; Rastogi, A. Distribution of Fluoride in Plasma, Brain, and Bones and Associated Oxidative Damage After Induced Chronic Fluorosis in Wistar Rats. Biol. Trace Elem. Res. 2022, 200, 1710–1721. [Google Scholar] [CrossRef]
- Peckham, S.; Awofeso, N. Water Fluoridation: A Critical Review of the Physiological Effects of Ingested Fluoride as a Public Health Intervention. Sci. World J. 2014, 2014, 293019. [Google Scholar] [CrossRef]
- Waugh, D.T. Fluoride Exposure Induces Inhibition of Sodium-and Potassium-Activated Adenosine Triphosphatase (Na+, K+-ATPase) Enzyme Activity: Molecular Mechanisms and Implications for Public Health. Int. J. Environ. Res. Public Health 2019, 16, 1427. [Google Scholar] [CrossRef]
- Goschorska, M.; Gutowska, I.; Baranowska-Bosiacka, I.; Piotrowska, K.; Metryka, E.; Safranow, K.; Chlubek, D. Influence of Acetylcholinesterase Inhibitors Used in Alzheimer’s Disease Treatment on the Activity of Antioxidant Enzymes and the Concentration of Glutathione in THP-1 Macrophages under Fluoride-Induced Oxidative Stress. Int. J. Environ. Res. Public Health 2018, 16, 10. [Google Scholar] [CrossRef]
- Khan, A.M.; Raina, R.; Dubey, N.; Verma, P.K. Effect of Deltamethrin and Fluoride Co-Exposure on the Brain Antioxidant Status and Cholinesterase Activity in Wistar Rats. Drug Chem. Toxicol. 2018, 41, 123–127. [Google Scholar] [CrossRef]
- Dharmaratne, R.W. Fluoride in Drinking Water and Diet: The Causative Factor of Chronic Kidney Diseases in the North Central Province of Sri Lanka. Environ. Health Prev. Med. 2015, 20, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Malin, A.J.; Lesseur, C.; Busgang, S.A.; Curtin, P.; Wright, R.O.; Sanders, A.P. Fluoride Exposure and Kidney and Liver Function among Adolescents in the United States: NHANES, 2013–2016. Environ. Int. 2019, 132, 105012. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Does Fluoride Cause the Mysterious Chronic Kidney Disease of Multifactorial Origin? Environ. Geochem. Health 2020, 42, 3035–3057. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, L.; Verma, P.K.; Raina, R.; Sood, S. Toxic Effects of Imidacloprid Combined with Arsenic: Oxidative Stress in Rat Liver. Toxicol. Ind. Health 2018, 34, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Baba, N.A.; Verma, P.K.; Sultana, M.; Singh, M. Hepatotoxicity Induced by Subchronic Exposure of Fluoride and Chlorpyrifos in Wistar Rats: Mitigating Effect of Ascorbic Acid. Biol. Trace Elem. Res. 2015, 166, 157–162. [Google Scholar] [CrossRef]
- Baba, N.A.; Raina, R.; Verma, P.K.; Sultana, M.; Prawez, S.; Nisar, N.A. Toxic Effects of Fluoride and Chlorpyrifos on Antioxidant Parameters in Rats: Protective Effects of Vitamins C and E. Fluoride 2013, 46, 73–79. [Google Scholar]
- Eugenio-Pérez, D.; Medina-Fernández, L.Y.; Saldivar-Anaya, J.A.; Molina-Jijón, E.; Pedraza-Chaverri, J. Role of Dietary Antioxidant Agents in Chronic Kidney Disease. In Free Radicals and Diseases; InTech: Singapore, 2016; ISBN 978-953-51-2747-5. [Google Scholar]
- Saafi-Ben Salah, E.B.; El Arem, A.; Louedi, M.; Saoudi, M.; Elfeki, A.; Zakhama, A.; Najjar, M.F.; Hammami, M.; Achour, L. Antioxidant-Rich Date Palm Fruit Extract Inhibits Oxidative Stress and Nephrotoxicity Induced by Dimethoate in Rat. J. Physiol. Biochem. 2012, 68, 47–58. [Google Scholar] [CrossRef]
- Iheka, C.U.; Onyegeme-Okerenta, B.M.; Anacletus, F.C. Impact of Fluoride Toxicity and Ameliorative Effects of Some Antioxidants on Selected Biochemical Indices of Male Rats. AASCIT J. Health 2015, 2, 87–92. [Google Scholar]
- Harborne, A.J. Phytochemical Methods A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 1998. [Google Scholar]
- FAO; WHO. Pesticide Residues in Food Evaluations Part II—Toxicological. Geneva, World Health Organization, Joint FAO/WHO Meeting on Pesticide Residues (WHO/PCS/97.1); FAO: Rome, Italy; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Sastry, K.V.H.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric Determination of Serum Nitrite and Nitrate by Copper-Cadmium Alloy. Analy. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef]
- Inkielewicz, I.; Krechniak, J. Fluoride Content in Soft Tissues and Urine of Rats Exposed to Sodium Fluoride in Drinking Water. Fluoride 2003, 36, 263–266. [Google Scholar]
- Burlina, A.; Michielin, E.; Galzigna, L. Characteristics and Behaviour of Arylesterase in Human Serum and Liver. Eur. J. Clin. Investig. 1977, 7, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Reduced Glutathione (GSH). In Red Blood Cell Metabolism a Manual of Biochemical Methods, 2nd ed.; Bergmeyen, H.V., Ed.; Grune and Stratton: New York, NY, USA, 1975; pp. 112–114. [Google Scholar]
- Mochnik, P.A.; Frei, B.; Ames, B.N. Measurement of Antioxidants in Human Blood Plasma. Methods Enzymol. 1994, 234, 269–279. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar]
- Hafeman, D.G.; Sunde, R.A.; Hoekstra, W.G. Effect of Dietary Selenium on Erythrocyte and Liver Glutathione Peroxidase in the Rat. J. Nutr. 1974, 104, 580–587. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione Reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef]
- Rehman, S.U. Lead-Induced Regional Lipid Peroxidation in Brain. Toxicol. Lett. 1984, 21, 333–337. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Pawar, A.P.; Sanaye, S.V.; Shyama, S.; Sreepada, R.A.; Dake, A.S. Effects of Salinity and Temperature on the Acute Toxicity of the Pesticides, Dimethoate and Chlorpyrifos in Post-Larvae and Juveniles of the Whiteleg Shrimp. Aquac. Rep. 2020, 16, 100240. [Google Scholar] [CrossRef]
- Karunarathne, A.; Bhalla, A.; Sethi, A.; Perera, U.; Eddleston, M. Importance of Pesticides for Lethal Poisoning in India during 1999 to 2018: A Systematic Review. BMC Public Health 2021, 21, 1441. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Podder, S.; Agarwal, S.; Bhattacharya, S. Fluoride-Induced Histopathology and Synthesis of Stress Protein in Liver and Kidney of Mice. Arch. Toxicol. 2011, 85, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, T.; Selvaraj, M. Sources of Human Overexposure to Fluoride, Its Toxicities, and Their Amelioration Using Natural Antioxidants. In Fluoride; IntechOpen: London, UK, 2022; ISBN 978-1-80355-643-7. [Google Scholar]
- Mahjoubi-Samet, A.; Fetoui, H.; Boujelben, G.; Jamoussi, K.; Ammar, E.; Ellouze, F.; Guermazi, F.; Zeghal, N. Effects of Dimethoate on Bone Maturation of Young Rats during the Suckling Period. Pestic. Biochem. Physiol. 2005, 83, 132–139. [Google Scholar] [CrossRef]
- Flora, S.J.S. Arsenic and Dichlorvos: Possible Interaction between Two Environmental Contaminants. J. Trace Elem. Med. Biol. 2016, 35, 43–60. [Google Scholar] [CrossRef]
- Mahajan, L.; Verma, P.K.; Raina, R.; Pankaj, N.K.; Sood, S.; Singh, M. Alteration in Thiols Homeostasis, Protein and Lipid Peroxidation in Renal Tissue Following Subacute Oral Exposure of Imidacloprid and Arsenic in Wistar Rats. Toxicol. Rep. 2018, 5, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Bashir, S.; Irshad, M.; Nag, T.C.; Dogra, T.D. Dimethoate-Induced Effects on Antioxidant Status of Liver and Brain of Rats Following Subchronic Exposure. Toxicology 2005, 215, 173–181. [Google Scholar] [CrossRef]
- Verma, P.K.; Singh, P.; Sharma, P.; Sood, S.; Raina, R. Dose-Dependent Oxidative Damage in Erythrocytes and Hepatic Tissue of Wistar Rats Concurrently Exposed with Arsenic and Quinalphos: A Subacute Study. Biol. Trace Elem. Res. 2022, 200, 2160–2173. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Forstermann, U.; Pollock, J.S.; Schmidt, H.H.H.W.; Heller, M.; Murad, F. Calmodulin-Dependent Endothelium-Derived Relaxing Factor/Nitric Oxide Synthase Activity Is Present in the Particulate and Cytosolic Fractions of Bovine Aortic Endothelial Cells. Proc. Natl. Acad. Sci. USA 1991, 88, 1788–1792. [Google Scholar] [CrossRef]
- Miranda, G.H.N.; Gomes, B.A.Q.; Bittencourt, L.O.; Aragão, W.A.B.; Nogueira, L.S.; Dionizio, A.S.; Buzalaf, M.A.R.; Monteiro, M.C.; Lima, R.R. Chronic Exposure to Sodium Fluoride Triggers Oxidative Biochemistry Misbalance in Mice: Effects on Peripheral Blood Circulation. Oxidative Med. Cell. Longev. 2018, 2018, 8379123. [Google Scholar] [CrossRef]
- Maheshwari, N.; Qasim, N.; Anjum, R.; Mahmood, R. Fluoride Enhances Generation of Reactive Oxygen and Nitrogen Species, Oxidizes Hemoglobin, Lowers Antioxidant Power and Inhibits Transmembrane Electron Transport in Isolated Human Red Blood Cells. Ecotoxicol. Environ. Saf. 2021, 208, 111611. [Google Scholar] [CrossRef]
- Sauerheber, R. Physiologic Conditions Affect Toxicity of Ingested Industrial Fluoride. J. Environ. Public Health 2013, 2013, 439490. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.R.; Strobel, S.A. Principles of Fluoride Toxicity and the Cellular Response: A Review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Rangoonwala, S.P.; Kazim, M.; Pandey, A.K. Effects of Diazinon on Serum Calcium and Inorganic Phosphate Levels as Well as Ultrastructures of Parathyroid and Calcitonin Cells of Rattus Norvegicus. J. Environ. Biol. 2005, 26, 217–221. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, K.; Ma, L.; Gu, H.; Li, J.; Lei, S. Fluoride Induced Endoplasmic Reticulum Stress and Calcium Overload in Ameloblasts. Arch. Oral Biol. 2016, 69, 95–101. [Google Scholar] [CrossRef]
- Shivarajashankara, Y.M.; Shivashankara, A.R.; Hanumanth Rao, S.; Gopalakrishna Bhat, P. Oxidative Stress in Children with Endemic Skeletal Fluorosis. Fluoride 2001, 34, 103–107. [Google Scholar]
- Verma, P.K.; Raina, R.; Sultana, M.; Singh, M.; Kumar, P. Total Antioxidant and Oxidant Status of Plasma and Renal Tissue of Cisplatin-Induced Nephrotoxic Rats: Protection by Floral Extracts of Calendula officinalis Linn. Ren. Fail. 2016, 38, 142–150. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, G.; Rubio-Escalante, F.J.; Noreña-Barroso, E.; Escalante-Herrera, K.S.; Schlenk, D. Impacts of Oxidative Stress on Acetylcholinesterase Transcription, and Activity in Embryos of Zebrafish (Danio Rerio) Following Chlorpyrifos Exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 172–173, 19–25. [Google Scholar] [CrossRef]
- Santi, A.; Menezes, C.; Duarte, M.; Leitemperger, J.; Lópes, T.; Loro, V. Oxidative Stress Biomarkers and Acetylcholinesterase Activity in Human Erythrocytes Exposed to Clomazone (In Vitro). Interdiscip. Toxicol. 2011, 4, 149–153. [Google Scholar] [CrossRef]
- Singh, P.; Verma, P.K.; Raina, R.; Sood, S.; Sharma, P. Maximum Contaminant Level of Arsenic in Drinking Water Potentiates Quinalphos-Induced Renal Damage on Co-Administration of Both Arsenic and Quinalphos in Wistar Rats. Environ. Sci. Pollut. Res. 2020, 27, 21331–21340. [Google Scholar] [CrossRef]
- Ludlow, M.; Luxton, G.; Mathew, T. Effects of Fluoridation of Community Water Supplies for People with Chronic Kidney Disease. Nephrol. Dial. Transplant. 2007, 22, 2763–2767. [Google Scholar] [CrossRef]
- Baba, N.; Raina, R.; Verma, P.; Sultana, M. Free Radical-Induced Nephrotoxicity Following Repeated Oral Exposure to Chlorpyrifos Alone and in Conjunction with Fluoride in Rats. Turk. J. Med. Sci. 2016, 46, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.M.; Muthumani, M. Silibinin Ameliorates Arsenic Induced Nephrotoxicity by Abrogation of Oxidative Stress, Inflammation and Apoptosis in Rats. Mol. Biol. Rep. 2012, 39, 11201–11216. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, M.; Jayabal, S.; Hemaiswarya, S.; Murugesan, S.; Enkateswara, S.; Doble, M.; Periyasamy, S. Polyphenol-rich Indian Ginger Cultivars Ameliorate GLUT4 Activity in C2C12 Cells, Inhibit Diabetes-related Enzymes and LPS-induced Inflammation: An in Vitro Study. J. Food Biochem. 2021, 45, e13600. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Faboya, O.L.; Paul, A.A.; Olayide, I.; Faboya, O.A.; Oluwasola, T.A. Nephroprotective Effect of Essential Oils from Ginger (Zingiber officinale) and Turmeric (Curcuma longa) Rhizomes against Cadmium-Induced Nephrotoxicity in Rats. J. Oleo Sci. 2018, 67, 1339–1345. [Google Scholar] [CrossRef]
- Gabr, S.A.; Alghadir, A.H.; Ghoniem, G.A. Biological Activities of Ginger against Cadmium-Induced Renal Toxicity. Saudi J. Biol. Sci. 2019, 26, 382–389. [Google Scholar] [CrossRef]
- Abaekwume, C.O.; Kagbo, H.D. Comparative Effect of Ginger (Zingiber officinale) Supplement on Hepato-Renal Damages Induced by Acetaminophen Toxicity in Wistar Rats. Asian J. Res. Med. Pharm. Sci. 2021, 10, 1–12. [Google Scholar] [CrossRef]
- Joshi, D.; Srivastav, S.K.; Belemkar, S.; Dixit, V.A. Zingiber officinale and 6-Gingerol Alleviate Liver and Kidney Dysfunctions and Oxidative Stress Induced by Mercuric Chloride in Male Rats: A Protective Approach. Biomed. Pharmacother. 2017, 91, 645–655. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Gol, A.; Dabiri, S.; Javadi, A. Preventive Effect of Ginger (Zingiber officinale) Pretreatment on Renal Ischemia-Reperfusion in Rats. Eur. Surg. Res. 2011, 46, 45–51. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, P.K.; Sood, S.; Yousuf, R.; Raina, R. Oxidative renal damage induced by fluoride and dimethoate and its mitigation by Zingiber officinale in Wistar rats. Res. Sq. 2022. [Google Scholar] [CrossRef]





| Time (min) | Water and 0.1% Acetic Acid | Acetonitrile |
|---|---|---|
| 0.01 | 95% | 5% |
| 5 | 95% | 5% |
| 15 | 10% | 90% |
| 20 | 10% | 90% |
| 25 | 95% | 5% |
| 30 | 95% | 5% |
| Groups | Treatment | Dose and Route of Administration |
|---|---|---|
| i. | Control | 1 mL/day/rat, per os (PO), drinking water |
| ii. | Zingiber officinale (ZO) | 300 mg/kg BW (body weight), PO |
| iii. | Dimethoate (DM) | 31.0 mg/kg BW (1/10th LD50), PO |
| iv. | Fluoride (F-) | 4.5 ppm in drinking water |
| v. | DM + F- | 1/10th of LD50 (PO) + 4.5 ppm in drinking water |
| vi. | ZO + DM | 300 mg/kg BW (PO) + 1/10th of LD50 (PO) |
| vii. | ZO + F- | 300 mg/kg BW (PO) + 4.5 ppm |
| viii. | ZO + DM + F- | 300 mg/kg BW (PO) + 1/10th of LD50 + 4.5 ppm |
| ix. | Quercetin + DM + F- | 100 mg/kg BW (PO) + 1/10th of LD50 + 4.5 ppm |
| Groups | BUN | CR | Uric Acid | Calcium | Phosphorus |
|---|---|---|---|---|---|
| Control | 42.84 a ± 2.40 | 0.43 a ± 0.03 | 2.57 a ± 0.41 | 10.99 c ± 0.56 | 9.72 c ± 0.47 |
| ZO Extract (300 mg/kg) | 38.54 a ± 3.64 | 0.49 a ± 0.07 | 3.49 a ± 0.97 | 10.34 c ± 0.93 | 8.28 ab ± 0.67 |
| Dimethoate (DM—1/10th LD50) | 56.45 b ± 4.42 | 1.11 c ± 0.12 | 4.52 bc ± 0.60 | 5.08 a ± 0.79 | 5.56 a ± 0.43 |
| Fluoride (F-—4.5 ppm) | 55.04 b ± 2.74 | 1.05 c ± 0.11 | 3.89 bc ± 0.49 | 5.55 a ± 0.53 | 6.70 b ± 0.81 |
| DM (1/10th LD50) + F- (4.5 ppm) | 112.99 d ± 3.69 | 1.50 d ± 0.08 | 4.82 c ± 0.47 | 5.21 a ± 0.84 | 5.32 a ± 0.59 |
| ZO Extract + DM (1/10th LD50) | 43.35 a ± 3.70 | 0.63 b ± 0.08 | 3.20 a ± 0.35 | 9.27 bc ± 0.43 | 9.27 abc ± 0.43 |
| ZO Extract + F- (4.5 ppm) | 44.44 a ± 3.14 | 0.60 b ± 0.08 | 3.30 ab ± 0.39 | 10.16 bc ± 0.32 | 10.16 bc ± 0.32 |
| ZO Extract + DM (1/10th LD50) + F- (4.5 ppm) | 81.37 c ± 6.58 | 0.53 ab ± 0.03 | 2.91 ab ± 0.58 | 12.40 c ± 0.79 | 10.40 c ± 0.79 |
| Quercetin (100 mg/kg) + DM (1/10th LD50) + F- (4.5 ppm) | 72.28 c ± 9.98 | 0.56 ab ± 0.02 | 3.19 b ± 0.52 | 10.63 cd ± 0.72 | 10.63 cd ± 0.72 |
| Groups | TAS | TTH | AE | AChE | CAT |
|---|---|---|---|---|---|
| Control | 20.26 a ± 0.35 | 1.96 c ± 0.42 | 3.41 b ± 0.36 | 17,241.00 c ± 890.71 | 3112.69 b ± 172.21 |
| ZO Extract (300 mg/kg) | 21.68 a ± 1.25 | 1.98 c ± 0.36 | 2.96 b ± 0.25 | 12,907.63 bc ± 448.89 | 3469.04 b ± 105.89 |
| Dimethoate (DM −1/10th LD50) | 16.01 b ± 0.19 | 1.07 b ± 0.28 | 0.80 a ± 0.06 | 8269.13 b ± 302.86 | 3072.02 b ± 166.92 |
| Fluoride (F-—4.5 ppm) | 15.12 b ± 1.13 | 1.25 ab ± 0.54 | 0.79 a ± 0.04 | 8158.13 b ± 709.49 | 3187.47 b ± 586.52 |
| DM (1/10th LD50) + F- (4.5 ppm) | 10.68 c ± 0.87 | 0.58 d ± 0.16 | 0.51 d ± 0.16 | 6574.50 a ± 434.04 | 1854.48 a ± 240.79 |
| ZO Extract (300 mg/kg) + DM (1/10th LD50) | 15.71 b ± 0.98 | 0.80 a ± 0.08 | 2.72 b ± 0.23 | 12,079.63 d ± 565.97 | 4372.77 d ± 898.00 |
| ZO Extract (300 mg/kg) + F- (4.5 ppm) | 16.13 b ± 1.49 | 1.19 a ± 0.54 | 2.70 b ± 0.40 | 14,446.50 e ± 442.64 | 2968.29 b ± 114.96 |
| ZO Extract (300 mg/kg) + DM (1/10th LD50) + F- (4.5 ppm) | 19.62 a ± 0.32 | 1.97 c ± 0.35 | 2.92 b ± 0.52 | 16,436.88 c ± 714.71 | 3276.49 b ± 133.96 |
| Quercetin (100 mg/kg) + DM (1/10th LD50) + F- (4.5 ppm) | 15.53 b ± 0.86 | 0.90 a ± 0.24 | 1.47 a ± 0.28 | 17,569.75 c ± 962.12 | 3007.47 b ± 490.10 |
| Groups | SOD | GPx | GR | AOPP | MDA |
|---|---|---|---|---|---|
| Control | 736.82 b ± 26.88 | 262.21 c ± 21.38 | 50.04 bc ± 3.26 | 1.42 a ± 0.14 | 42.03 a ± 4.55 |
| ZO Extract (300 mg/kg) | 869.32 b ± 16.46 | 273.16 c ± 13.08 | 47.30 b ± 3.41 | 1.51 a ± 0.05 | 52.15 a ± 8.98 |
| Dimethoate (DM −1/10th LD50) | 348.06 a ± 22.81 | 125.63 a ± 6.78 | 27.99 b ± 3.47 | 1.75 b ± 0.27 | 315.69 c ± 20.35 |
| Fluoride (F-—4.5 ppm) | 329.59 a ± 21.55 | 128.66 a ± 5.72 | 17.26 a ± 1.74 | 1.87 b ± 0.21 | 385.97 c ± 48.91 |
| DM (1/10th LD50) + F- (4.5 ppm) | 252.88 c ± 11.52 | 193.67 a ± 1.46 | 14.52 a ± 1.49 | 2.65 d ± 0.32 | 492.83 d ± 15.35 |
| ZO Extract (300 mg/kg) + DM (1/10th LD50) | 705.58 b ± 35.99 | 252.26 bc ± 29.04 | 36.17 a ± 4.05 | 1.49 a ± 0.05 | 159.95 b ± 9.64 |
| ZO Extract (300 mg/kg) + F- (4.5 ppm) | 781.95 b ± 35.79 | 232.84 b ± 18.99 | 45.12 bc ± 12.74 | 1.38 a ± 0.10 | 158.08 b ± 9.52 |
| ZO Extract (300 mg/kg) + DM (1/10th LD50) + F- (4.5 ppm) | 738.11 b ± 24.28 | 205.83 ab ± 13.99 | 52.58 bc ± 6.87 | 1.44 a ± 0.04 | 155.74 b ± 8.06 |
| Quercetin (100 mg/kg) + DM (1/10th LD50) + F- (4.5 ppm) | 921.80 d ± 32.34 | 197.89 ab ± 14.14 | 27.14 a ± 2.22 | 1.46 a ± 0.13 | 66.80 e ± 7.84 |
| Treatments | Lesion Scores in Different Groups | ||||||
|---|---|---|---|---|---|---|---|
| Congestion | Intertubular Hemorrhage | Edema | Casts | Tubular Degeneration | Tubular Necrosis | Glomerular Degeneration | |
| Control | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ZO Extract (300 mg/kg) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Dimethoate (DM −1/10th LD50) | 1.83 * | 0.00 | 0.00 | 1.33 * | 2.00 * | 0.00 | 1.17 * |
| Fluoride (F-—4.5 ppm) | 1.83 * | 1.67 * | 1.17 * | 1.00 * | 1.67 * | 0.67 * | 0.67 * |
| DM (1/10th LD50) + F- (4.5 ppm) | 2.50 ** | 2.33 ** | 2.33 ** | 2.17 ** | 2.50 ** | 2.17 ** | 2.67 ** |
| ZO Extract (300 mg/kg) + DM (1/10th LD50) | 1.00 * | 0.00 | 0.00 | 0.83 * | 1.00 * | 0.00 | 1.17 * |
| ZO Extract (300 mg/kg) + F- (4.5 ppm) | 1.00 * | 1.00 * | 0.00 | 0.83 * | 1.00 * | 0.00 | 1.17 * |
| ZO Extract (300 mg/kg) + DM (1/10th LD50) + F- (4.5 ppm) | 0.67 * | 0.00 | 0.00 | 1.00 | 0.50 * | 0.00 | 0.83 * |
| Quercetin (100 mg/kg) + DM (1/10th LD50) + F- (4.5 ppm) | 0.83 * | 1.33 * | 1.17 * | 0.83 * | 1.67 * | 0.83 * | 0.83 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Verma, P.K.; Sood, S.; Yousuf, R.; Kumar, A.; Raina, R.; Shabbir, M.A.; Bhat, Z.F. Protective Effect of Quercetin and Ginger (Zingiber officinale) Extract against Dimethoate Potentiated Fluoride-Induced Nephrotoxicity in Rats. Foods 2023, 12, 1899. https://doi.org/10.3390/foods12091899
Sharma P, Verma PK, Sood S, Yousuf R, Kumar A, Raina R, Shabbir MA, Bhat ZF. Protective Effect of Quercetin and Ginger (Zingiber officinale) Extract against Dimethoate Potentiated Fluoride-Induced Nephrotoxicity in Rats. Foods. 2023; 12(9):1899. https://doi.org/10.3390/foods12091899
Chicago/Turabian StyleSharma, Priyanka, Pawan Kumar Verma, Shilpa Sood, Rasia Yousuf, Amit Kumar, Rajinder Raina, Muhammad Asim Shabbir, and Zuhaib F. Bhat. 2023. "Protective Effect of Quercetin and Ginger (Zingiber officinale) Extract against Dimethoate Potentiated Fluoride-Induced Nephrotoxicity in Rats" Foods 12, no. 9: 1899. https://doi.org/10.3390/foods12091899
APA StyleSharma, P., Verma, P. K., Sood, S., Yousuf, R., Kumar, A., Raina, R., Shabbir, M. A., & Bhat, Z. F. (2023). Protective Effect of Quercetin and Ginger (Zingiber officinale) Extract against Dimethoate Potentiated Fluoride-Induced Nephrotoxicity in Rats. Foods, 12(9), 1899. https://doi.org/10.3390/foods12091899