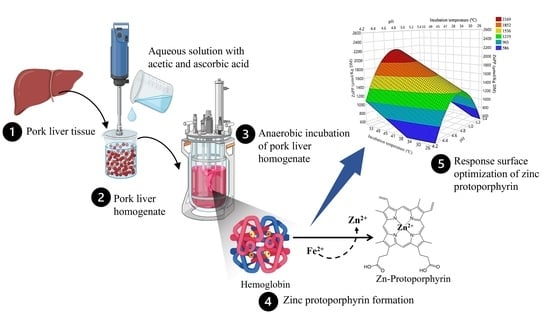
Utilization of Porcine Livers through the Formation of Zn-Protoporphyrin Pigment Optimized by a Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Materials
Standard Procedure for the Preparation of Porcine Liver Homogenates
2.3. Effect of the Addition of Disulfite and Organic Acids
2.4. Response Surface Methodology (RSM)
2.4.1. Experimental Design
2.4.2. Validation of the Optimal ZnPP-Forming Conditions
2.5. Porphyrin Determination
2.6. Challenge Test with Pathogenic Bacteria
2.7. Microbiological Analysis
2.8. Zinc-Chelatase Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of the Addition of Disulfite and Organic Acids on ZnPP Formation and Microbial Growth
3.2. Optimization of ZnPP Formation Using RSM
3.2.1. Selection and Validation of Optimal Conditions for ZnPP Formation
3.2.2. Definition and Safety Aspects of the Potential Coloring Ingredient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Sullivan, M.G.; Kerry, J.P. Sensory Evaluation of Fresh Meat. In Improving the Sensory and Nutritional Quality of Fresh Meat; Elsevier: Amsterdam, The Netherlands, 2009; pp. 178–196. [Google Scholar] [CrossRef]
- Skibsted, L.H. Nitric Oxide and Quality and Safety of Muscle Based Foods. Nitric Oxide 2011, 24, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Toldrá, F. Chemistry, Safety, and Regulatory Considerations in the Use of Nitrite and Nitrate from Natural Origin in Meat Products—Invited Review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef] [PubMed]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-Nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential. Mutat. Res. Toxicol. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary Nitrate and Nitrite: Benefits, Risks, and Evolving Perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef]
- Bou, R.; Llauger, M.; Arnau, J.; Olmos, A.; Fulladosa, E. Formation of Zn-Protoporphyrin during the Elaboration Process of Non-Nitrified Serrano Dry-Cured Hams and Its Relationship with Lipolysis. Food Chem. 2022, 374, 131730. [Google Scholar] [CrossRef]
- Wakamatsu, J.; Nishimura, T.; Hattori, A. A Zn–Porphyrin Complex Contributes to Bright Red Color in Parma Ham. Meat Sci. 2004, 67, 95–100. [Google Scholar] [CrossRef]
- Adamsen, C.E.; Møller, J.K.S.; Hismani, R.; Skibsted, L.H. Thermal and Photochemical Degradation of Myoglobin Pigments in Relation to Colour Stability of Sliced Dry-Cured Parma Ham and Sliced Dry-Cured Ham Produced with Nitrite Salt. Eur. Food Res. Technol. 2004, 218, 403–409. [Google Scholar] [CrossRef]
- Kauser-Ul-Alam, M.; Toba, Y.; Hioki, S.; Hayakawa, T.; Kumura, H.; Wakamatsu, J. Lactococcus lactis Subsp. Cremoris Produces Zinc Protoporphyrin IX Both Aerobically and Anaerobically and Improves the Bright Red Color of Fermented Meat Products. Foods 2020, 9, 1583. [Google Scholar] [CrossRef]
- Benedini, R.; Raja, V.; Parolari, G. Zinc-Protoporphyrin IX Promoting Activity in Pork Muscle. LWT—Food Sci. Technol. 2008, 41, 1160–1166. [Google Scholar] [CrossRef]
- Wakamatsu, J. Evidence of the Mechanism Underlying Zinc Protoporphyrin IX Formation in Nitrite/Nitrate-Free Dry-Cured Parma Ham. Meat Sci. 2022, 192, 108905. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C. Ferrochelatase. Int. J. Biochem. Cell Biol. 1999, 31, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.T.; Ishigaki, M.; Kataoka, T.; Taketani, S. Ferrochelatase Catalyzes the Formation of Zn-Protoporphyrin of Dry-Cured Ham via the Conversion Reaction from Heme in Meat. J. Agric. Food Chem. 2011, 59, 12238–12245. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Kawabuchi, T.; Kawakami, Y.; Sato, M.; Numata, M.; Matsumoto, K. Formation of Zinc Protoporphyrin IX and Protoporphyrin IX from Oxymyoglobin in Porcine Heart Mitochondria. Food Sci. Technol. Res. 2007, 13, 85–88. [Google Scholar] [CrossRef]
- De Maere, H.; Chollet, S.; Claeys, E.; Michiels, C.; Govaert, M.; De Mey, E.; Paelinck, H.; Fraeye, I. In Vitro Zinc Protoporphyrin IX Formation in Different Meat Sources Related to Potentially Important Intrinsic Parameters. Food Bioprocess Technol. 2017, 10, 131–142. [Google Scholar] [CrossRef]
- Wakamatsu, J.; Akter, M.; Honma, F.; Hayakawa, T.; Kumura, H.; Nishimura, T. Optimal pH of Zinc Protoporphyrin IX Formation in Porcine Muscles: Effects of Muscle Fiber Type and Myoglobin Content. LWT 2019, 101, 599–606. [Google Scholar] [CrossRef]
- Wakamatsu, J.; Murakami, N.; Nishimura, T. A Comparative Study of Zinc Protoporphyrin IX-Forming Properties of Animal by-Products as Sources for Improving the Color of Meat Products. Anim. Sci. J. 2015, 86, 547–552. [Google Scholar] [CrossRef]
- Ockerman, H.W.; Hansen, C.L. Animal by-Product Processing & Utilization, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9781566767774. [Google Scholar]
- Honikel, K.-O. Composition and Calories. In Handbook of Analysis of Edible Animal by-Products; Nollet, L.M.L., Toldrà, F., Eds.; Taylor & Francis Group: Abingdon, UK, 2011; pp. 105–122. ISBN 9780429148262. [Google Scholar]
- Silva, R.O.; Rouxinol, M.I.F.C.; Patarata, L.A.S.C. Pork Liver Freshness Evaluated through Spoilage Microbiota and a Consumer Test in Shelf Life Extension Experiment. J. Food Qual. 2020, 2020, 1092865. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Hereu, A.; Bover-Cid, S.; Garriga, M.; Aymerich, T. High Hydrostatic Pressure and Biopreservation of Dry-Cured Ham to Meet the Food Safety Objectives for Listeria monocytogenes. Int. J. Food Microbiol. 2012, 154, 107–112. [Google Scholar] [CrossRef]
- Duncan, C.L.; Strong, D.H. Improved Medium for Sporulation of Clostridium Perfringens. Appl. Microbiol. 1968, 16, 82–89. [Google Scholar] [CrossRef]
- Labbe, R.G.; Rey, D.K. Raffinose Increases Sporulation and Enterotoxin Production by Clostridium Perfringens Type A. Appl. Environ. Microbiol. 1979, 37, 1196–1200. [Google Scholar] [CrossRef]
- Parolari, G.; Benedini, R.; Toscani, T. Color Formation in Nitrite-Free Dried Hams as Related to Zn-Protoporphyrin IX and Zn-Chelatase Activity. J. Food Sci. 2009, 74, C413–C418. [Google Scholar] [CrossRef] [PubMed]
- Dailey, H.A.; Dailey, T.A.; Wu, C.-K.K.; Medlock, A.E.; Rose, J.P.; Wang, K.-F.F.; Rose, J.P.; Wang, B.-C. Ferrochelatase at the Millennium: Structures, Mechanisms and [2Fe-2S] Clusters. Cell. Mol. Life Sci. 2000, 57, 1909–1926. [Google Scholar] [CrossRef]
- Taketani, S.; Ishigaki, M.; Mizutani, A.; Uebayashi, M.; Numata, M.; Ohgari, Y.; Kitajima, S. Heme Synthase (Ferrochelatase) Catalyzes the Removal of Iron from Heme and Demetalation of Metalloporphyrins. Biochemistry 2007, 46, 15054–15061. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, J.; Okui, J.; Hayashi, N.; Nishimura, T.; Hattori, A. Zn Protoporphyrin IX Is Formed Not from Heme but from Protoporphyrin IX. Meat Sci. 2007, 77, 580–586. [Google Scholar] [CrossRef]
- Tong, Y.; Guo, M. Bacterial Heme-Transport Proteins and Their Heme-Coordination Modes. Arch. Biochem. Biophys. 2009, 481, 1–15. [Google Scholar] [CrossRef]
- Baureder, M.; Hederstedt, L. Heme Proteins in Lactic Acid Bacteria, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 62, ISBN 9780124105157. [Google Scholar] [CrossRef]
- Wakamatsu, J.; Kawazoe, H.; Ohya, M.; Hayakawa, T.; Kumura, H. Improving the Color of Meat Products without Adding Nitrite/Nitrate Using High Zinc Protoporphyrin IX-Forming Microorganisms. Meat Sci. 2020, 161, 107989. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, J.R.; Reuter, R.J.; Morton, K.O.; Wehner, J.M. Enzymatic Formation of Zinc-Protoporphyrin by Rat Liver and Its Potential Effect on Hepatic Heme Metabolism. Gastroenterology 1983, 85, 663–668. [Google Scholar] [CrossRef]
- Chau, T.T.; Ishigaki, M.; Kataoka, T.; Taketani, S. Porcine Ferrochelatase: The Relationship between Iron-Removal Reaction and the Conversion of Heme to Zn-Protoporphyrin. Biosci. Biotechnol. Biochem. 2010, 74, 1415–1420. [Google Scholar] [CrossRef]
- Gao, Z.; Shao, J.; Sun, H.; Zhong, W.; Zhuang, W.; Zhang, Z. Evaluation of Different Kinds of Organic Acids and Their Antibacterial Activity in Japanese Apricot Fruits. Afr. J. Agric. Res. 2012, 7, 4911–4918. [Google Scholar] [CrossRef]
- Selim, S.A.; El Alfy, S.M.; Abdel Aziz, M.H.; Mashait, M.S.; Warrad, M.F. Evolution of Bactericidal Activity of Selected Food Additives against Food Borne Microbial Pathogens. Biosci. Biotechnol. Res. Asia 2012, 9, 7–17. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Yoshida, T.; Kikuchi, G. Features of the Reaction of Heme Degradation Catalyzed by the Reconstituted Microsomal Heme Oxygenase System. J. Biol. Chem. 1978, 253, 4230–4236. [Google Scholar] [CrossRef] [PubMed]
- De Maere, H.; Fraeye, I.; De Mey, E.; Dewulf, L.; Michiels, C.; Paelinck, H.; Chollet, S. Formation of Naturally Occurring Pigments during the Production of Nitrite-Free Dry Fermented Sausages. Meat Sci. 2016, 114, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Shiraishi, A.; Kumura, H.; Hayakawa, T.; Wakamatsu, J. Investigation of Contributors to Zinc Protoporphyrin IX Formation at Optimum pH 5.5 in Pork. Anim. Sci. J. 2019, 90, 774–780. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Ohya, M.; Kumura, H.; Hayakawa, T.; Wakamatsu, J. Searching for High ZnPP-Forming Edible Bacteria to Improve the Color of Fermented Meat Products without Nitrite/Nitrate. Meat Sci. 2020, 165, 108109. [Google Scholar] [CrossRef]
- Khozroughi, A.G.; Kroh, L.W.; Schlüter, O.; Rawel, H. Assessment of the Bacterial Impact on the Post-Mortem Formation of Zinc Protoporphyrin IX in Pork Meat. Food Chem. 2018, 256, 25–30. [Google Scholar] [CrossRef]
- Hanna, M.O.; Smith, G.C.; Savell, J.W.; Mckeith, F.K.; Vanderzant, C. Microbial Flora of Livers, Kidneys and Hearts from Beef, Pork and Lamb: Effects of Refrigeration, Freezing and Thawing. J. Food Prot. 1982, 45, 63–73. [Google Scholar] [CrossRef]
- Medlock, A.E.; Dailey, H.A. New Avenues of Heme Synthesis Regulation. Int. J. Mol. Sci. 2022, 23, 7467. [Google Scholar] [CrossRef]
- Schultz, I.J.; Chen, C.; Paw, B.H.; Hamza, I. Iron and Porphyrin Trafficking in Heme Biogenesis. J. Biol. Chem. 2010, 285, 26753–26759. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Gorris, L.G.M. Hurdle Technology. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 221–227. ISBN 9780123847331. [Google Scholar] [CrossRef]
- Bou, R.; Llauger, M.; Arnau, J.; Fulladosa, E. Zinc-Protoporphyrin Content in Commercial Parma Hams is Affected by Proteolysis Index and Marbling. Meat Sci. 2018, 139, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Bustins, N.; Martín, B.; Llauger, M.; Bou, R.; Bover-Cid, S.; Jofré, A. Dynamics of Microbial Communities in Nitrite-Free and Nutritionally Improved Dry Fermented Sausages. Fermentation 2023, 9, 403. [Google Scholar] [CrossRef]
- Khozroughi, A.G.; Jander, E.; Schirrmann, M.; Rawel, H.; Kroh, L.W.; Schlüter, O. The Role of Myoglobin Degradation in the Formation of Zinc Protoporphyrin IX in the Longissimus Lumborum of Pork. LWT—Food Sci. Technol. 2017, 85, 22–27. [Google Scholar] [CrossRef]
- Khozroughi, A.G.; Braga, T.W.; Wagner, J.; Rawel, H. Investigation of the Post Mortem Zinc Protoporphyrin IX Fluorescence with Respect to Its Protein-Bound and Unbound Occurrence in Aqueous Meat Extracts. Food Chem. 2019, 283, 462–467. [Google Scholar] [CrossRef] [PubMed]
- International Commission of Microbiological Specifications for Foods (ICMSF). Microorganisms in Foods 5. Characteristics of Microbial Pathogens, 1st ed.; Springer: New York, NY, USA, 1996; ISBN 978-0-412-47350-0. [Google Scholar]
- Mejlholm, O.; Dalgaard, P. Development and Validation of an Extensive Growth and Growth Boundary Model for Listeria monocytogenes in Lightly Preserved and Ready-to-Eat Shrimp. J. Food Prot. 2009, 72, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Beier, R.C.; Callaway, T.R.; Andrews, K.; Poole, T.L.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Interactions of Organic Acids with Salmonella Strains from Feedlot Water-Sprinkled Cattle. J. Food Chem. Nanotechnol. 2017, 03, 60–66. [Google Scholar] [CrossRef]
- Leistner, L. Basic Aspects of Food Preservation by Hurdle Technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef] [PubMed]
 , total viable counts of aerobic mesophilic bacteria (TVC)
, total viable counts of aerobic mesophilic bacteria (TVC)  , and Zn-protoporphyrin (ZnPP) content
, and Zn-protoporphyrin (ZnPP) content  . Liver homogenates (20%, w/w) were adjusted to pH 4.5 and incubated anaerobically at 37 °C in the dark for 24 h. The concentrations of ZnPP and heme are expressed on a dry-weight basis (% DM). TVC is expressed as log CFU/mL homogenate. Bars represent the standard error (n = 3). Significance differences among addition levels within the same incubation time are indicated by different letters (p < 0.05).
. Liver homogenates (20%, w/w) were adjusted to pH 4.5 and incubated anaerobically at 37 °C in the dark for 24 h. The concentrations of ZnPP and heme are expressed on a dry-weight basis (% DM). TVC is expressed as log CFU/mL homogenate. Bars represent the standard error (n = 3). Significance differences among addition levels within the same incubation time are indicated by different letters (p < 0.05).
 , total viable counts of aerobic mesophilic bacteria (TVC)
, total viable counts of aerobic mesophilic bacteria (TVC)  , and Zn-protoporphyrin (ZnPP) content
, and Zn-protoporphyrin (ZnPP) content  . Liver homogenates (20%, w/w) were adjusted to pH 4.5 and incubated anaerobically at 37 °C in the dark for 24 h. The concentrations of ZnPP and heme are expressed on a dry-weight basis (% DM). TVC is expressed as log CFU/mL homogenate. Bars represent the standard error (n = 3). Significance differences among addition levels within the same incubation time are indicated by different letters (p < 0.05).
. Liver homogenates (20%, w/w) were adjusted to pH 4.5 and incubated anaerobically at 37 °C in the dark for 24 h. The concentrations of ZnPP and heme are expressed on a dry-weight basis (% DM). TVC is expressed as log CFU/mL homogenate. Bars represent the standard error (n = 3). Significance differences among addition levels within the same incubation time are indicated by different letters (p < 0.05).

| Factors | Levels | ||||
|---|---|---|---|---|---|
| −1.68 | −1 | 0 | +1 | +1.68 | |
| Temperature (°C) | 25 | 31 | 40 | 49 | 55 |
| pH | 4.2 | 4.4 | 4.8 | 5.2 | 5.4 |
| Time (h) | 3 | 8.5 | 16.5 | 24.5 | 30 |
| Run | Temperature (°C) 1 | pH 1 | Time (h) 1 | Heme (µmol/kg) 2 | ZnPP (µmol/kg) 2 | TVC (Log CFU/mL) 2 |
|---|---|---|---|---|---|---|
| 1 | 55 (50) | 4.80 (4.79) | 16.5 (17.0) | 515 ± 7 | 2195 ± 20 | 2.85 ± 0.01 |
| 2 | 40 (37) | 4.20 (4.23) | 16.5 (16.6) | 310 ± 14 | 643 ± 1 | 3.14 ± 0.14 |
| 3 | 40 (37) | 4.80 (4.79) | 16.5 (16.7) | 318 ± 12 | 1754 ± 51 | 3.23 ± 0.05 |
| 4 | 40 (37) | 4.80 (4.79) | 3.0 (3.2) | 333 ± 51 | 617 ± 9 | 2.97 ± 0.02 |
| 5 | 40 (37) | 4.80 (4.79) | 16.5 (16.7) | 358 ± 13 | 1829 ± 13 | 3.13 ± 0.05 |
| 6 | 40 (37) | 5.40 (5.41) | 16.5 (16.7) | 382 ± 17 | 788 ± 4 | 6.18 ± 0.21 |
| 7 | 31 (32) | 5.20 (5.21) | 8.5 (8.7) | 357 ± 16 | 1006 ± 15 | 2.95 ± 0.05 |
| 8 | 31 (32) | 5.20 (5.21) | 24.5 (25.0) | 1422 ± 21 | 1380 ± 11 | 5.26 ± 0.14 |
| 9 | 40 (37) | 4.80 (4.79) | 30.0 (30.0) | 940 ± 22 | 2538 ± 73 | 3.39 ± 0.25 |
| 10 | 49 (47) | 5.20 (5.21) | 8.5 (8.3) | 335 ± 86 | 1185 ± 52 | 2.87 ± 0.09 |
| 11 | 49 (47) | 5.20 (5.21) | 24.5 (25.0) | 1258 ± 30 | 1699 ± 45 | 2.95 ± 0.01 |
| 12 | 49 (47) | 4.40 (4.40) | 8.5 (8.3) | 381 ± 21 | 1347 ± 42 | 3.02 ± 0.32 |
| 13 | 40 (37) | 4.80 (4.79) | 16.5 (16.7) | 499 ± 153 | 1728 ± 22 | 3.22 ± 0.04 |
| 14 | 31 (32) | 4.40 (4.40) | 24.5 (25.0) | 1067 ± 17 | 1798 ± 25 | 3.16 ± 0.01 |
| 15 | 25 (25) | 4.80 (4.79) | 16.5 (17.0) | 617 ± 143 | 1200 ±16 | 3.54 ± 0.01 |
| 16 | 49 (47) | 4.40 (4.40) | 24.5 (25.0) | 1157 ± 1 | 1468 ± 1 | 2.77 ± 0.07 |
| 17 | 31 (32) | 4.40 (4.40) | 8.5 (8.7) | 330 ± 5 | 513 ± 25 | 3.20 ± 0.12 |
| Term | Degrees of Freedom | Sum of Squares | F | Coefficient | Standard Error | t Value | p-Value | Standard Beta |
|---|---|---|---|---|---|---|---|---|
| Constant | 1755.930 | 53.9589 | 32.543 | <0.0001 | 0.000 | |||
| Incubation time (t) | 1 | 3585.966 | 78.97 | −366.049 | 41.1925 | −8.886 | <0.0001 | −0.602 |
| Incubation temperature (T°C) | 1 | 1166.106 | 25.68 | −242.202 | 47.795 | −5.067 | <0.0001 | −0.336 |
| t · T°C | 1 | 463.142 | 10.20 | −189.832 | 59.441 | −3.194 | 0.0034 | −0.217 |
| pH2 | 1 | 3357.405 | 73.94 | −350.978 | 40.818 | −8.599 | <0.0001 | −0.569 |
| Model | 4 | 9083.892 | 50.06 | <0.0001 | ||||
| Pure Error | 12 | 32.931 | ||||||
| Total | 29 | 1325.367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llauger, M.; Arnau, J.; Albano-Gaglio, M.; Bover-Cid, S.; Martín, B.; Bou, R. Utilization of Porcine Livers through the Formation of Zn-Protoporphyrin Pigment Optimized by a Response Surface Methodology. Foods 2023, 12, 1903. https://doi.org/10.3390/foods12091903
Llauger M, Arnau J, Albano-Gaglio M, Bover-Cid S, Martín B, Bou R. Utilization of Porcine Livers through the Formation of Zn-Protoporphyrin Pigment Optimized by a Response Surface Methodology. Foods. 2023; 12(9):1903. https://doi.org/10.3390/foods12091903
Chicago/Turabian StyleLlauger, Mar, Jacint Arnau, Michela Albano-Gaglio, Sara Bover-Cid, Belén Martín, and Ricard Bou. 2023. "Utilization of Porcine Livers through the Formation of Zn-Protoporphyrin Pigment Optimized by a Response Surface Methodology" Foods 12, no. 9: 1903. https://doi.org/10.3390/foods12091903
APA StyleLlauger, M., Arnau, J., Albano-Gaglio, M., Bover-Cid, S., Martín, B., & Bou, R. (2023). Utilization of Porcine Livers through the Formation of Zn-Protoporphyrin Pigment Optimized by a Response Surface Methodology. Foods, 12(9), 1903. https://doi.org/10.3390/foods12091903