Circular Economy and Sustainable Recovery of Taiwanese Tilapia (Oreochromis mossambicus) Byproduct—The Large-Scale Production of Umami-Rich Seasoning Material Application
Abstract
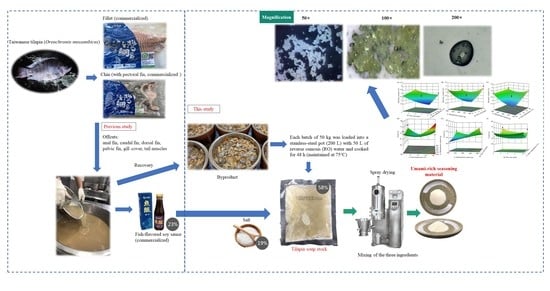
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Processing of Samples
2.3. Experimental Design of Response Surface Methodology (RSM) for the Umami-Rich Seasoning
2.4. Basic Composition Analysis
2.5. Physicochemical Analysis
2.6. Free Amino Acids
2.7. Determination of Water Solubility Index (WSI)
2.8. Bulk and Tapped Densities
2.9. Fourier-Transform Infrared Spectroscopy (FTIR)
2.10. Dynamic Optical Microscope Photography Technology-Confocal
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Compositions
3.2. Optimal Formulation and Conditions Using RSM for the Umami-Rich Seasoning
3.2.1. Selection of Carriers
3.2.2. RSM
3.3. Physicochemical Characterization of Umami-Rich Seasoning Powder
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Independent Variables | Run | Coded | Uncoded |
|---|---|---|---|
| A: Maltodextrin content (g) | ±1 | 1 | 500 |
| ±1 | 0 | 400 | |
| 0 | −1 | 300 | |
| 0 | |||
| B: Flow rate (rpm) | ±1 | 1 | 6 |
| 0 | 0 | 4 | |
| ±1 | −1 | 2 | |
| 0 | |||
| C: Inlet temperature (°C) | 0 | 1 | 80 |
| ±1 | 0 | 70 | |
| ±1 | −1 | 60 | |
| 0 | |||
| Number of runs | 15 |
| Tilapia | |||
|---|---|---|---|
| Soup Stock | Cooking Liquid 1 | Concentrated Liquid 2 | |
| Crude protein (%) | 2.8 ± 0.01 a | 4.8 ± 0.01 b | 11.6 ± 0.01 c |
| Crude fat (%) | 0.2 ± 0.01 a | N.D. | 0.5 ± 0.05 b |
| Moisture (%) | 92.8 ± 0.02 a | 95.0 ± 0.02 b | 88.0 ± 1.00 c |
| Ash (%) | 0.1 ± 0.02 a | 0.4 ± 0.02 b | 1.1 ± 1.05 c |
| Sugar content (°Brix) | 3.33 ± 0.12 a | 6.93 ± 0.09 b | 14.27 ± 0.09 c |
| Salinity (g/100 g) | 3.00 ± 0.00 a | 5.73 ± 0.09 b | 12.47 ± 0.09 c |
| pH value | 6.42 ± 0.31 a | 6.06 ± 0.01 b | 5.99 ± 0.00 c |
| L* | 0.90 ± 0.00 a | 4.31 ± 0.15 b | 0.57 ± 0.04 c |
| a* | 0.47 ± 0.00 a | 0.62 ± 0.19 b | 0.31 ± 0.18 c |
| b* | 1.56 ± 0.00 a | 4.19 ± 0.07 b | 0.98 ± 0.08 c |
| ΔE | 0.00 ± 0.00 a | 0.42 ± 0.01 b | 0.43 ± 0.00 c |
| L* | a* | b* | Price (USD/25 Kg) | |
|---|---|---|---|---|
| Maltodextrin | 86.10 ± 0.73 b | 1.35 ± 0.16 a | 13.77 ± 0.46 b | 48.82 c |
| Lactose | 88.61 ± 0.06 a | 0.99 ± 0.11 b | 11.63 ± 0.07 c | 81.36 b |
| Indigestible dextrin | 84.68 ± 0.22 c | 1.21 ± 0.65 a | 15.46 ± 0.39 a | 136.69 a |
| Source | Sum of Squares | DF | Mean | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Square | ||||||
| Model | 6.84 | 9 | 0.7599 | 5.67 | 0.0352 | Significant |
| A: Maltodextrin content | 0.726 | 1 | 0.726 | 5.42 | 0.0673 | |
| B: Flow rate | 1.58 | 1 | 1.58 | 11.77 | 0.0186 | |
| C: Inlet temperature | 1.8 | 1 | 1.8 | 13.48 | 0.0144 | |
| AB | 0.8836 | 1 | 0.8836 | 6.6 | 0.0501 | |
| AC | 0.5256 | 1 | 0.5256 | 3.93 | 0.1044 | |
| BC | 0.024 | 1 | 0.024 | 0.1795 | 0.6894 | |
| A2 | 1.11 | 1 | 1.11 | 8.27 | 0.0348 | |
| B2 | 0.2642 | 1 | 0.2642 | 1.97 | 0.219 | |
| C2 | 0.0299 | 1 | 0.0299 | 0.2234 | 0.6563 | |
| Residual | 0.6693 | 5 | 0.1339 | |||
| Lack of fit | 0.4047 | 3 | 0.1349 | 1.02 | 0.5298 | Not significant |
| Pure error | 0.2646 | 2 | 0.1323 | |||
| Cor Total | 7.51 | 14 | ||||
| Statistical data | ||||||
| Std Dev. | 0.3659 | |||||
| Mean | 4.77 | |||||
| C.V.% | 7.67 | |||||
| R2 | 0.9109 | |||||
| Adjusted R2 | 0.7504 | |||||
| Predicted R2 | 0.0583 | |||||
| Adeq precision | 6.9169 | |||||
| Run | A | B | C | Drying | |||
|---|---|---|---|---|---|---|---|
| Maltodextrin Content | Flow Rate | Inlet Temperature | Before | After | Yield | Moisture Content | |
| Unit | g | rpm | °C | g | g | % | % |
| 1 | 400 | 6 | 60 | 600 | 446 | 74.33 | 4.96 |
| 2 | 300 | 4 | 60 | 500 | 324 | 64.8 | 5.31 |
| 3 | 400 | 4 | 70 | 600 | 392 | 65.33 | 4.08 |
| 4 | 500 | 6 | 70 | 700 | 509 | 72.71 | 5.13 |
| 5 | 400 | 2 | 60 | 600 | 460 | 76.67 | 5.33 |
| 6 | 300 | 4 | 80 | 500 | 370 | 74 | 3.68 |
| 7 | 400 | 4 | 70 | 600 | 400 | 66.67 | 4.71 |
| 8 | 500 | 2 | 70 | 700 | 479 | 68.43 | 5.43 |
| 9 | 400 | 2 | 80 | 600 | 399 | 66.5 | 4.49 |
| 10 | 300 | 6 | 70 | 500 | 304.5 | 60.9 | 3.84 |
| 11 | 500 | 4 | 80 | 700 | 503 | 71.86 | 5.27 |
| 12 | 400 | 4 | 70 | 600 | 402 | 67 | 4.53 |
| 13 | 500 | 4 | 60 | 700 | 535 | 76.43 | 5.45 |
| 14 | 400 | 6 | 80 | 600 | 413 | 68.83 | 3.81 |
| 15 | 300 | 2 | 70 | 500 | 357.5 | 71.5 | 6.03 |



References
- Fraga-Corral, M.; Ronza, P.; Garcia-Oliveira, P.; Pereira, A.G.; Losada, A.P.; Prieto, M.A.; Quiroga, M.I.; Simal-Gandara, J. Aquaculture as a circular bio-economy model with Galicia as a study case: How to transform waste into revalorized by-products. Trends Food Sci. Technol. 2022, 119, 23–35. [Google Scholar] [CrossRef]
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Venugopal, V. Valorization of seafood processing discards: Bioconversion and bio-refinery approaches. Front. Sustain. Food Syst. 2021, 5, 611835. [Google Scholar] [CrossRef]
- Zabochnicka, M.; Krzywonos, M.; Romanowska-Duda, Z.; Szufa, S.; Darkalt, A.; Mubashar, M. Algal biomass utilization toward circular economy. Life 2022, 12, 1480. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Luan, J.; Zhu, W.; Xu, Y.; Yi, S.; Li, J.; Wang, J.; Li, X. Effects of ultrasound pretreatment at different powers on flavor characteristics of enzymatic hydrolysates of cod (Gadus macrocephalus) head. Food Res. Int. 2022, 159, 111612. [Google Scholar] [CrossRef]
- Sajib, M.; Trigo, J.P.; Abdollahi, M.; Undeland, I. Pilot-scale ensilaging of herring filleting co-products and subsequent separation of fish oil and protein hydrolysates. Food Bioprocess Technol. 2022, 15, 2267–2281. [Google Scholar] [CrossRef]
- Muscolo, A.; Mauriello, F.; Marra, F.; Calabrò, P.S.; Russo, M.; Ciriminna, R.; Pagliaro, M. AnchoisFert: A new organic fertilizer from fish processing waste for sustainable agriculture. Glob. Chall. 2022, 6, 2100141. [Google Scholar] [CrossRef]
- de la Fuente, B.; Pinela, J.; Mandim, F.; Heleno, S.A.; Ferreira, I.C.F.R.; Barba, F.J.; Berrada, H.; Caleja, C.; Barros, L. Nutritional and bioactive oils from salmon (Salmo salar) side streams obtained by Soxhlet and optimized microwave-assisted extraction. Food Chem. 2022, 386, 132778. [Google Scholar] [CrossRef]
- Lucarini, M.; Zuorro, A.; Di Lena, G.; Lavecchia, R.; Durazzo, A.; Benedetti, B.; Lombardi-Boccia, G. Sustainable management of secondary raw materials from the marine food-chain: A case-study perspective. Sustainability 2020, 12, 8997. [Google Scholar] [CrossRef]
- Sasidharan, A.; Venugopal, V. Proteins and co-products from seafood processing discards: Their recovery, functional properties and applications. Waste Biomass Valorization 2020, 11, 5647–5663. [Google Scholar] [CrossRef]
- Kruijssen, F.; Tedesco, I.; Ward, A.; Pincus, L.; Love, D.; Thorne-Lyman, A.L. Loss and waste in fish value chains: A review of the evidence from low and middle-income countries. Glob. Food Secur. 2020, 26, 100434. [Google Scholar] [CrossRef]
- Do, Q.; Mishra, N.; Colicchia, C.; Creazza, A.; Ramudhin, A. An extended institutional theory perspective on the adoption of circular economy practices: Insights from the seafood industry. Int. J. Prod. Econ. 2022, 247, 108400. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis (OMA) of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Hou, C.-Y.; Lin, C.-M.; Patel, A.K.; Dong, C.; Shih, M.-K.; Hsieh, C.-W.; Hung, Y.-L.; Huang, P.-H. Development of novel green methods for preparation of lead-free preserved pidan (duck egg). J. Food Sci. Technol. 2022, 60, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.P.; Calado, R.; Ameixa, O.M.C.C.; Valcarcel, J.; Vázquez, J.A. Valorisation of Atlantic codfish (Gadus morhua) frames from the cure-salting industry as fish protein hydrolysates with in vitro bioactive properties. LWT 2021, 149, 111840. [Google Scholar] [CrossRef]
- Huang, P.-H.; Chiu, C.-S.; Lu, W.-C.; Li, P.-H. Effect of compositions on physicochemical properties and rheological behavior of gelatinized adzuki-bean cake (Yokan). LWT 2022, 168, 113870. [Google Scholar] [CrossRef]
- Xu, Y.X.; Dzenis, Y.; Hanna, M.A. Water solubility, thermal characteristics and biodegradability of extruded starch acetate foams. Ind. Crops Prod. 2005, 21, 361–368. [Google Scholar] [CrossRef]
- Amidon, G.E.; Meyer, P.J.; Mudie, D.M. Chapter 10—Particle, powder, and compact characterization. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 271–293. [Google Scholar] [CrossRef]
- Coucoulas, L. Agglomeration. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 73–80. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Budziak-Wieczorek, I.; Czernel, G.; Karcz, D.; Barańska, A.; Jedlińska, A.; Samborska, K. Classification of honey powder composition by FTIR spectroscopy coupled with chemometric analysis. Molecules 2022, 27, 3800. [Google Scholar] [CrossRef]
- Huang, P.-H.; Hazeena, S.H.; Qiu, Y.-T.; Ciou, J.-Y.; Hsieh, C.-W.; Shih, M.-K.; Chen, M.-H.; Hou, C.-Y. Application of egg white hydrolysate (EWH) to improve frothing functionality of pasteurized liquid egg in large quantity production. Heliyon 2023, 9, e12697. [Google Scholar] [CrossRef]
- Peng, K.; Fu, B.; Li, J.; Zhao, H.; Cao, J.; Huang, W.; Chen, B.; Li, X.; Peng, Z.; Wei, M. Effects of replacing soybean meal and rapeseed meal with faba bean meal on growth performance and muscle quality of tilapia (Oreochromis niloticus). Aquac. Rep. 2022, 26, 101328. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; López-Martínez, L.A.; González-García, R.; Martínez, J.O.; Compeán Martínez, I.; Toxqui-Terán, A. Evaluation of the spray drying conditions of blueberry juice-maltodextrin on the yield, content, and retention of quercetin 3-d-galactoside. Polymers 2019, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.A.; Calderas, F.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Ochoa-Martínez, L.A.; Bernad-Bernad, M.J. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT-Food Sci. Technol. 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Fernandez Rivas, D.; Cintas, P. On an intensification factor for green chemistry and engineering: The value of an operationally simple decision-making tool in process assessment. Sustain. Chem. Pharm. 2022, 27, 100651. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Shahgol, M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A by spray-drying, using binary and ternary blends of gum arabic, starch and maltodextrin. Food Hydrocoll. 2020, 108, 106029. [Google Scholar] [CrossRef]
- Rosales-Chimal, S.; Navarro-Cortez, R.O.; Bello-Perez, L.A.; Vargas-Torres, A.; Palma-Rodríguez, H.M. Optimal conditions for anthocyanin extract microencapsulation in taro starch: Physicochemical characterization and bioaccessibility in gastrointestinal conditions. Int. J. Biol. Macromol. 2023, 227, 83–92. [Google Scholar] [CrossRef]
- Baltrusch, K.L.; Torres, M.D.; Domínguez, H.; Flórez-Fernández, N. Spray-drying microencapsulation of tea extracts using green starch, alginate or carrageenan as carrier materials. Int. J. Biol. Macromol. 2022, 203, 417–429. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Azhar, M.D.; Hashib, S.A.; Ibrahim, U.K.; Rahman, N.A. Development of carrier material for food applications in spray drying technology: An overview. Mater. Today Proc. 2021, 47, 1371–1375. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Pereira, R.R.; Carvalho-Guimarães, F.B.d.; Remígio, M.S.d.N.; Barbosa, W.L.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. Microencapsulation by spray drying and antioxidant activity of phenolic compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) almonds. Polymers 2022, 14, 2905. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of spray drying microencapsulation of almond oil into taro starch spherical aggregates. LWT 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Liang, L.; Zhou, C.; Zhang, J.; Huang, Y.; Zhao, J.; Sun, B.; Zhang, Y. Characteristics of umami peptides identified from porcine bone soup and molecular docking to the taste receptor T1R1/T1R3. Food Chem. 2022, 387, 132870. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Xi, Y.; Shi, Y.; Qiu, Z.; Matsuoka, R.; Wang, H.; Xu, C.; Tao, N.; Zhang, L.; Wang, X. Effects of temperature fluctuations on non-volatile taste compounds in tilapia fillets (Oreochromis niloticus). Food Chem. 2023, 408, 135227. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; Liu, Y. Characterization and evaluation of umami taste: A review. TrAC Trends Anal. Chem. 2020, 127, 115876. [Google Scholar] [CrossRef]
- Li, M.; Chung, S.-J. Flavor principle as an implicit frame: Its effect on the acceptance of instant noodles in a cross-cultural context. Food Qual. Prefer. 2021, 93, 104293. [Google Scholar] [CrossRef]
- Sun, L.-B.; Zhang, Z.-Y.; Xin, G.; Sun, B.-X.; Bao, X.-J.; Wei, Y.-Y.; Zhao, X.-M.; Xu, H.-R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Marcus, J.B. Chapter 6—Flavor enhancement ingredients. In Aging, Nutrition and Taste; Marcus, J.B., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 173–206. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.P.; Ayed, C.; Li, B.; Liu, Y. An on-line study about consumers’ perception and purchasing behavior toward umami seasonings in China. Food Control 2020, 110, 107037. [Google Scholar] [CrossRef]
- Hayabuchi, H.; Morita, R.; Ohta, M.; Nanri, A.; Matsumoto, H.; Fujitani, S.; Yoshida, S.; Ito, S.; Sakima, A.; Takase, H.; et al. Validation of preferred salt concentration in soup based on a randomized blinded experiment in multiple regions in Japan-influence of umami (L-glutamate) on saltiness and palatability of low-salt solutions. Hypertens. Res. 2020, 43, 525–533. [Google Scholar] [CrossRef]
- Xie, X.; Dang, Y.; Pan, D.; Sun, Y.; Zhou, C.; He, J.; Gao, X. The enhancement and mechanism of the perception of saltiness by umami peptide from Ruditapes philippinarum and ham. Food Chem. 2023, 405, 134886. [Google Scholar] [CrossRef]
- An, F.; Cao, K.; Ji, S.; Wang, Y.; Pan, G.; Ma, Y.; Zhao, Y.; Wu, J.; Wu, R. Identification, taste characterization, and molecular docking study of a novel microbiota-derived umami peptide. Food Chem. 2023, 404, 134583. [Google Scholar] [CrossRef]
- Ferrell, C.M.; Lauf, P.K.; Wilson, B.A.; Adragna, N.C. Lithium and protein kinase C modulators regulate swelling-activated K-Cl cotransport and reveal a complete phosphatidylinositol cycle in low K sheep erythrocytes. J. Membr. Biol. 2000, 177, 81–93. [Google Scholar] [CrossRef]
- Wallace, T.C.; Cowan, A.E.; Bailey, R.L. Current sodium intakes in the United States and the modeled effects of glutamate incorporation into select savory products. Nutrients 2019, 11, 2691. [Google Scholar] [CrossRef]
- Maluly, H.D.B.; Arisseto-Bragotto, A.P.; Reyes, F.G.R. Monosodium glutamate as a tool to reduce sodium in foodstuffs: Technological and safety aspects. Food Sci. Nutr. 2017, 5, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Mukherjee, S.; Maji, B.K. Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: An overview. Toxicol. Rep. 2021, 8, 938–961. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-C.; Li-Chan, E.C.Y.; Jao, C.-L. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011, 126, 617–622. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Sadeghi Mahoonak, A.R.; Rafipour, F. Development of animal/ plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Venugopal, V. Chapter three—Enzymes from seafood processing waste and their applications in seafood processing. In Advances in Food and Nutrition Research; Kim, S.-K., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 78, pp. 47–69. [Google Scholar]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S.; Maqsood, S. Valorization of fish byproducts: Sources to end-product applications of bioactive protein hydrolysate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1803–1842. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Bogusławska-Wąs, E.; Drozłowska, E.; Trocer, P.; Dłubała, A.; Mazurkiewicz-Zapałowicz, K.; Bartkowiak, A. The application of spray-dried and reconstituted flaxseed oil cake extract as encapsulating material and carrier for probiotic Lacticaseibacillus rhamnosus GG. Materials 2021, 14, 5324. [Google Scholar] [CrossRef]
- Svečnjak, L.; Bubalo, D.; Baranović, G.; Novosel, H. Optimization of FTIR-ATR spectroscopy for botanical authentication of unifloral honey types and melissopalynological data prediction. Eur. Food Res. Technol. 2015, 240, 1101–1115. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR spectroscopy to the quantification of sugar in honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef]
- Anguebes, F.; Pat, L.; Ali, B.; Guerrero, A.; Córdova, A.V.; Abatal, M.; Garduza, J.P. Application of multivariable analysis and FTIR-ATR spectroscopy to the prediction of properties in Campeche honey. J. Anal. Methods Chem. 2016, 2016, 5427526. [Google Scholar] [CrossRef]
- Elzey, B.; Pollard, D.; Fakayode, S.O. Determination of adulterated neem and flaxseed oil compositions by FTIR spectroscopy and multivariate regression analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Welti-Chanes, J. Chilled foods: Effects on shelf-life and sensory quality. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 14–18. [Google Scholar] [CrossRef]
- Chun, S.; Chambers, E.; Chambers, D.H. Effects of shiitake (Lentinus edodes P.) mushroom powder and sodium tripolyphosphate on texture and flavor of pork patties. Foods 2020, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Chambers, V.E.; Tran, T.; Chambers IV, E. Natural: A $75 billion word with no definition—Why not? J. Sens. Stud. 2019, 34, e12501. [Google Scholar] [CrossRef]



| Run | Salinity (g/100 g) | Sugar Content (%) | pH | Moisture Content (%) | AW | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 73.00 ± 0.14 h | 97.00 ± 0.14 bc | 5.15 ± 0.00 h | 4.96 ± 0.06 d | 0.36 ± 0.01 cd | 92.70 ±0.69 d | 0.82 ± 0.03 c | 5.46 ± 0.14 b | 92.87 ± 0.69 e |
| 2 | 84.00 ± 0.00 abc | 100.0 ± 0.00 a | 4.99 ± 0.02 i | 5.31 ± 0.06 b | 0.40 ± 0.01 a | 92.54 ± 0.23 D | 1.07 ± 0.01 ab | 16.07 ± 0.12 a | 92.74 ± 0.23 e |
| 3 | 83.00 ± 0.14 bcd | 97.00 ± 0.05 bc | 5.40 ± 0.05 ef | 4.08 ± 0.11 f | 0.37 ± 0.01 c | 93.26 ± 0.39 cd | 1.04 ± 0.06 ab | 7.11 ± 0.10 b | 93.53 ± 0.40 cde |
| 4 | 83.00 ± 0.14 bcd | 97.00 ± 0.14 bc | 5.43 ± 0.03 cde | 5.13 ± 0.06 c | 0.40 ± 0.03 ab | 94.51 ±0.32 b | 0.64 ± 0.03 d | 10.06 ± 0.02 ab | 95.05 ± 0.32 ab |
| 5 | 86.00 ± 0.03 ab | 100.0 ± 0.03 a | 5.48 ± 0.03 abc | 5.33 ± 0.08 b | 0.36 ± 0.00 c | 95.72 ±0.50 a | 0.83 ± 0.04 c | 3.40 ± 0.03 b | 95.78 ± 0.49 a |
| 6 | 85.33 ± 0.03 abc | 99.30 ± 0.03 ab | 5.27 ± 0.03 g | 3.68 ± 0.17 g | 0.28 ± 0.01 g | 91.09 ±0.17 e | 1.09 ± 0.03 a | 4.15 ± 0.03 b | 91.19 ± 0.17 f |
| 7 | 78.00 ± 0.00 de | 92.00 ± 0.04 ef | 5.51 ± 0.04 ab | 4.71 ± 0.03 e | 0.33 ± 0.01 e | 90.09 ± 0.34 efg | 0.04 ± 0.02 g | 7.81 ± 0.10 ab | 90.43 ± 0.33 f |
| 8 | 80.00 ± 0.00 de | 94.00 ± 0.00 de | 5.44 ± 0.03 a | 5.43 ± 0.06 b | 0.37 ± 0.01 c | 94.49 ± 0.12 b | 0.17 ± 0.03 f | 3.63 ± 0.10 b | 94.56 ± 0.12 bc |
| 9 | 82.00 ± 0.00 cd | 96.00 ± 0.00 cd | 5.44 ± 0.00 cde | 4.49 ± 0.03 e | 0.33 ± 0.01 e | 93.20 ± 0.44 cd | 0.67 ± 0.14 d | 5.85 ± 0.21 b | 93.38 ± 0.43 de |
| 10 | 77.00 ± 0.14 efg | 91.00 ± 0.14 f | 5.42 ± 0.02 de | 3.84 ± 0.04 g | 0.30 ± 0.01 g | 87.93 ± 0.29 h | 0.97 ± 0.13 b | 10.62 ± 0.23 ab | 88.57 ± 0.31 g |
| 11 | 65.33 ± 0.12 i | 79.33 ± 0.12 h | 5.41 ± 0.04 def | 5.27 ± 0.14 bc | 0.32 ± 0.02 ef | 93.92 ± 0.92 bc | 0.07 ± 0.07 g | 3.55 ± 0.03 b | 93.90 ± 0.91 cd |
| 12 | 76.00 ± 0.23 fgh | 93.33 ± 0.23 ef | 5.45 ± 0.00 cde | 4.53 ± 0.21 e | 0.29 ± 0.00 g | 90.60 ± 0.29 efg | −0.24 ± 0.02 i | 8.39 ± 0.07 ab | 90.99 ± 0.28 f |
| 13 | 86.00 ± 0.00 ab | 100.0 ± 0.00 a | 5.41 ± 0.04 def | 5.45 ± 0.13 a | 0.34 ± 0.00 de | 89.09 ± 0.83 g | −0.83 ± 0.03 j | 4.75 ± 0.02 b | 89.22 ± 0.83 g |
| 14 | 74.00 ± 0.00 gh | 88.00 ± 0.00 g | 5.46 ± 0.05 bcd | 3.81 ± 0.09 g | 0.30 ± 0.02 fg | 90.73 ± 0.32 ef | 0.46 ± 0.05 e | 10.46 ± 0.09 ab | 91.34 ± 0.33 f |
| 15 | 87.00 ± 0.14 a | 100.0 ± 0.14 a | 5.36 ± 0.01 f | 6.03 ± 0.02 a | 0.38 ± 0.03 bc | 89.96 ± 0.37 fg | −0.07 ± 0.04 h | 7.43 ± 0.20 b | 90.27 ± 0.38 f |
| mg/100 g | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspartic acid | 0.14 | 0.14 | 0.12 | 0.11 | 0.09 | 0.12 | 0.12 | 0.06 | 0.10 | 0.13 | 0.08 | 0.12 | 0.09 | 0.12 | 0.12 |
| Threonine | 0.06 | 0.06 | 0.05 | 0.05 | 0.04 | 0.05 | 0.05 | 0.03 | 0.04 | 0.05 | 0.04 | 0.05 | 0.04 | 0.05 | 0.05 |
| Serine | 0.07 | 0.07 | 0.06 | 0.05 | 0.04 | 0.05 | 0.06 | 0.03 | 0.05 | 0.06 | 0.04 | 0.06 | 0.05 | 0.05 | 0.06 |
| Glutamic acid | 0.29 | 0.29 | 0.25 | 0.22 | 0.18 | 0.24 | 0.25 | 0.12 | 0.20 | 0.26 | 0.17 | 0.25 | 0.19 | 0.23 | 0.24 |
| Glycine | 0.12 | 0.12 | 0.11 | 0.10 | 0.08 | 0.11 | 0.11 | 0.06 | 0.09 | 0.12 | 0.07 | 0.11 | 0.08 | 0.10 | 0.10 |
| Alanine | 0.11 | 0.11 | 0.10 | 0.09 | 0.07 | 0.10 | 0.10 | 0.05 | 0.08 | 0.10 | 0.06 | 0.10 | 0.07 | 0.09 | 0.09 |
| Cystamine | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Valine | 0.07 | 0.07 | 0.06 | 0.05 | 0.04 | 0.06 | 0.06 | 0.03 | 0.05 | 0.06 | 0.04 | 0.06 | 0.04 | 0.06 | 0.06 |
| Methionine | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Isoleucine | 0.05 | 0.05 | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | 0.02 | 0.04 | 0.05 | 0.03 | 0.04 | 0.03 | 0.04 | 0.04 |
| Leucine | 0.09 | 0.09 | 0.07 | 0.06 | 0.05 | 0.07 | 0.07 | 0.04 | 0.06 | 0.08 | 0.05 | 0.07 | 0.05 | 0.06 | 0.07 |
| Tyrosine | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Phenylalanine | 0.05 | 0.06 | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | 0.02 | 0.04 | 0.05 | 0.03 | 0.04 | 0.04 | 0.05 | 0.04 |
| Lysine | 0.11 | 0.12 | 0.10 | 0.08 | 0.06 | 0.10 | 0.10 | 0.04 | 0.09 | 0.11 | 0.07 | 0.10 | 0.08 | 0.10 | 0.09 |
| Tryptophan | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Histamine | 0.03 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.11 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| Arginine | 0.07 | 0.07 | 0.06 | 0.05 | 0.04 | 0.06 | 0.06 | 0.03 | 0.05 | 0.06 | 0.04 | 0.06 | 0.04 | 0.05 | 0.06 |
| Proline | 0.11 | 0.09 | 0.06 | 0.05 | 0.03 | 0.05 | 0.06 | 0.02 | 0.07 | 0.09 | 0.05 | 0.06 | 0.04 | 0.05 | 0.07 |
| Sample | WSI (%) | Density | |
|---|---|---|---|
| Bulk (g/cm3) | Tapped (g/mL) | ||
| 1 | 99.68 ± 0.17 a | 0.63 ± 0.01 b | 0.68 ± 0.01 a |
| 2 | 99.77 ± 0.16 a | 0.67 ± 0.02 a | 0.69 ± 0.02 a |
| 3 | 99.54 ± 0.65 a | 0.57 ± 0.01 c | 0.60 ± 0.01 c |
| 4 | 99.74 ± 0.20 a | 0.44 ± 0.02 de | 0.48 ± 0.01 de |
| 5 | 99.77 ± 0.09 a | 0.44 ± 0.01 de | 0.48 ± 0.01 d |
| 6 | 98.46 ± 0.84 b | 0.56 ± 0.02 c | 0.63 ± 0.01 b |
| 7 | 98.35 ± 0.16 b | 0.41 ± 0.02 ef | 0.44 ± 0.03 f |
| 8 | 99.86 ± 0.00 a | 0.41 ± 0.01 ef | 0.44 ± 0.01 f |
| 9 | 99.89 ± 0.06 a | 0.41 ± 0.01 ef | 0.44 ± 0.02 f |
| 10 | 99.78 ± 0.17 a | 0.40 ± 0.01 f | 0.43 ± 0.01 f |
| 11 | 99.75 ± 0.01 a | 0.43 ± 0.03 def | 0.46 ± 0.03 def |
| 12 | 99.80 ± 0.03 a | 0.45 ± 0.02 d | 0.49 ± 0.01 d |
| 13 | 99.87 ± 0.04 a | 0.41 ± 0.00 ef | 0.48 ± 0.01 de |
| 14 | 99.89 ± 0.03 a | 0.42 ± 0.01 def | 0.48 ± 0.01 ef |
| 15 | 99.79 ± 0.06 a | 0.41 ± 0.03 ef | 0.45 ± 0.03 bc |
| Commercially available seasoning powder (Bonito soup stock) | 89.70 ± 1.15 c | - | |
| Maltodextrin | 97.75 ± 0.31 cb | - | |
| Sample | Appearance | Flavor | ||||||
|---|---|---|---|---|---|---|---|---|
| Color | Odor | Umami | Sweetness | Salty | Mouthfeel | Overall Preference | ||
| Umami-rich seasoning powder | 7.67 ± 0.98 b | 7.33 ± 0.72 a | 7.53 ± 0.99 a | 7.40 ± 1.12 a | 7.60 ± 0.99 a | 7.47 ± 0.99 a | 7.53 ± 0.83 a | |
| Commercially available seasoning powder | 1 | 7.07 ± 1.22 b | 7.13 ± 1.64 a | 7.00 ± 2.00 a | 6.87 ± 1.92 b | 6.73 ± 1.91 a | 6.40 ± 2.06 a | 6.73 ± 1.33 a |
| 2 | 8.07 ± 0.80 a | 7.20 ± 0.68 a | 7.80 ± 0.86 a | 7.07 ± 1.53 c | 7.53 ± 0.99 a | 7.53 ± 1.06 a | 7.47 ± 0.99 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Huang, Y.-T.; Ciou, J.-Y.; Cheng, C.-M.; Wang, G.-T.; You, C.-M.; Huang, P.-H.; Hou, C.-Y. Circular Economy and Sustainable Recovery of Taiwanese Tilapia (Oreochromis mossambicus) Byproduct—The Large-Scale Production of Umami-Rich Seasoning Material Application. Foods 2023, 12, 1921. https://doi.org/10.3390/foods12091921
Lin C-H, Huang Y-T, Ciou J-Y, Cheng C-M, Wang G-T, You C-M, Huang P-H, Hou C-Y. Circular Economy and Sustainable Recovery of Taiwanese Tilapia (Oreochromis mossambicus) Byproduct—The Large-Scale Production of Umami-Rich Seasoning Material Application. Foods. 2023; 12(9):1921. https://doi.org/10.3390/foods12091921
Chicago/Turabian StyleLin, Chia-Hua, Ying-Tang Huang, Jhih-Ying Ciou, Chiu-Min Cheng, Guan-Ting Wang, Chun-Mei You, Ping-Hsiu Huang, and Chih-Yao Hou. 2023. "Circular Economy and Sustainable Recovery of Taiwanese Tilapia (Oreochromis mossambicus) Byproduct—The Large-Scale Production of Umami-Rich Seasoning Material Application" Foods 12, no. 9: 1921. https://doi.org/10.3390/foods12091921
APA StyleLin, C. -H., Huang, Y. -T., Ciou, J. -Y., Cheng, C. -M., Wang, G. -T., You, C. -M., Huang, P. -H., & Hou, C. -Y. (2023). Circular Economy and Sustainable Recovery of Taiwanese Tilapia (Oreochromis mossambicus) Byproduct—The Large-Scale Production of Umami-Rich Seasoning Material Application. Foods, 12(9), 1921. https://doi.org/10.3390/foods12091921