Strategies to Assess the Impact of Sustainable Functional Food Ingredients on Gut Microbiota
Abstract
:1. Introduction
2. Functional Ingredients and Sustainability
2.1. Fortified Foods
2.2. Food Fortification within a Circular Economy Framework
3. Gut Microbiota—A Perspective on Fortified Food Properties
4. Methods Available to Evaluate Human Gut Microbiota Modulation
| Technique | Description | Function | -Omic | References |
|---|---|---|---|---|
| Culture | Isolation of bacteria on selective media | To quantify culturable viable bacteria present in biological samples | Culturomics | [66,71] |
| Quantitative polymerase chain reaction (qPCR) | Amplification and quantification of 16S rRNA. Reaction mixture contains a compound that fluoresces after binding to double-stranded DNA | To identify and quantify the presence of a specific microorganism in biological samples | Metagenomics | [71,75,76] |
| Denaturing or temperature gradient gel electrophoresis (DGGE)/(TGGE) | Chemical or temperature denaturation and gel separation of 16 rRNA amplicons | To characterize microbial communities and their functional genes in biological samples | ||
| Fluorescence in situ hybridization (FISH) | Hybridization of fluorescent labeled oligonucleotide probes with target 16S rRNA complementary sequences. This approach can be coupled with a special microscope or to flow cytometry to enumerate the number of fluorescence events | To identify and quantify the presence of specific live microorganisms in biological samples | ||
| Microbiome shotgun sequencing | Random break-up of the whole genome into small DNA fragments followed by parallel sequencing of each fragment. A computer program analyzes the results of the DNA sequences to reconstitute the whole genome. | To determine the DNA sequences of the whole genome in the biological samples. To characterize, identify, and quantify the microbial communities present in the biological sample | ||
| High-performance liquid chromatography (HPLC) | Chemical separation of components in a liquid mixture. The liquid sample is injected into a pressurized liquid solvent (mobile phase) that goes through a column packed with a separation medium (stationary phase). Each component present in the sample interacts with the stationary phase, separating by a process of differential migration during the time spent travelling through the column. This process is monitored by a computerized system of detectors. | To identify and quantify specific metabolites present in biological samples (e.g., SCFAs) | Metabolomics | [77,78] |
| Gas chromatography (GC) | Chemical separation of components in a liquid or gaseous mixture. The liquid or gaseous sample is injected into a carrier gas (mobile phase) that goes through a column (stationary phase). The column is inside of an oven that regulates the temperature of the carrier gas and the eluent that leaves the column. This process is monitored by a computerized system of detectors. |
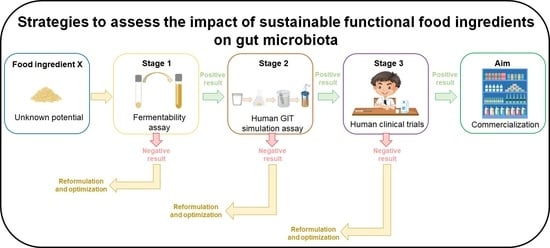
5. Proposed Strategy to Assess the Impact of Fortified Foods on the Gut Microbiota
5.1. Stage 1—Fermentabilty Assay
5.2. Stage 2—Human GIT Simulation Model
5.3. Stage 3—Human Clinical Trials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dable-Tupas, G.; Otero, M.C.B.; Bernolo, L. Functional Foods and Health Benefits. In Functional Foods and Nutraceuticals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–11. [Google Scholar]
- Kiousi, D.E.; Chorianopoulos, N.; Tassou, C.C.; Galanis, A. The Clash of Microbiomes: From the Food Matrix to the Host Gut. Microorganisms 2022, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Commission, C.A. Guidelines for Use of Nutrition and Health Claims (CAC/GL 23-1997 as Last Amended 2013). Rome: World Health Organization and the Food and Agriculture Organization of the United Nations, 2013. Br. J. Nutr. 2011, 106, 1231–1239. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Cuamatzin-García, L.; Rodríguez-Rugarcía, P.; El-Kassis, E.G.; Galicia, G.; Meza-Jiménez, M.d.L.; Baños-Lara, M.; Zaragoza-Maldonado, D.S.; Pérez-Armendáriz, B. Traditional Fermented Foods and Beverages from around the World and Their Health Benefits. Microorganisms 2022, 10, 1151. [Google Scholar] [CrossRef]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad, S.M.I. Interactions of Probiotics and Prebiotics with the Gut Microbiota. Prog. Mol. Biol. Transl. Sci. 2020, 171, 265–300. [Google Scholar]
- Alexandri, M.; Kachrimanidou, V.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products. Foods 2022, 11, 2796. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The Effects of Inulin on Gut Microbial Composition: A Systematic Review of Evidence from Human Studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-Thinking Functional Food Development through a Holistic Approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Alpizar-Vargas, L.; Zúñiga-Montero, C.; Rodríguez-Murillo, A.; Vargas-Vásquez, A.; Vega-Baudrit, J. Nutraceuticals: Definition, Applied Nanoengineering in Their Production and Applications. Int. J. Biosen Bioelectron. 2019, 5, 56–61. [Google Scholar]
- Reque, P.M.; Brandelli, A. Encapsulation of Probiotics and Nutraceuticals: Applications in Functional Food Industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- De Sousa, C.S.; Hassan, S.S.; Pinto, A.C.; Silva, W.M.; De Almeida, S.S.; Soares, S.D.C.; Azevedo, M.S.P.; Rocha, C.S.; Barh, D.; Azevedo, V. Microbial Omics: Applications in Biotechnology. In Omics Technologies and Bio-Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–20. [Google Scholar]
- He, M.; Tan, C.P.; Liu, Y.; Xu, Y.-J. Foodomics: A New Perspective on Gut Probiotics Nutrition and Health Research. Curr. Opin. Food Sci. 2021, 41, 146–151. [Google Scholar] [CrossRef]
- Settachaimongkon, S.; van Valenberg, H.J.F.; Smid, E.J. Metabolomics as an Emerging Strategy for the Investigation of Yogurt Components. In Yogurt in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 427–449. [Google Scholar]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Tiwari, A.; Khawas, R. Food Waste and Agro By-Products: A Step towards Food Sustainability. In Innovation in the Food Sector through the Valorization of Food and Agro-Food By-Products; IntechOpen: Rijeka, Croatia, 2021; ISBN 1838806830. [Google Scholar]
- Dorin, Ţ.; Melinda, F. Sustainable and Healthy Food Ingredients: Characterization and Application in Functional Products. In Functional Foods-Phytochemicals and Health Promoting Potential; IntechOpen: Rijeka, Croatia, 2021; ISBN 1839689331. [Google Scholar]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Agro-Industrial Fruit Byproducts as Health-Promoting Ingredients Used to Supplement Baked Food Products. Foods 2022, 11, 3181. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Functional Ingredients from Agri-Food Waste: Effect of Inclusion Thereof on Phenolic Compound Content and Bioaccessibility in Bakery Products. Antioxidants 2020, 9, 1216. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, A.D.; Melgar, B.; Conidi, C.; Barros, L.; Ferreira, I.C.F.R.; Cassano, A.; Garcia-Castello, E.M. Food Industry By-Products Valorization and New Ingredients: Cases of Study. In Sustainability of the Food System; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–99. [Google Scholar]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Amin, K.; Akhtar, S.; Ismail, T. Nutritional and Organoleptic Evaluation of Functional Bread Prepared from Raw and Processed Defatted Mango Kernel Flour. J. Food Process. Preserv. 2018, 42, e13570. [Google Scholar] [CrossRef]
- Plazzotta, S.; Sillani, S.; Manzocco, L. Exploitation of Lettuce Waste Flour to Increase Bread Functionality: Effect on Physical, Nutritional, Sensory Properties and on Consumer Response. Int. J. Food Sci. Technol. 2018, 53, 2290–2297. [Google Scholar] [CrossRef]
- Prokopov, T.; Chonova, V.; Slavov, A.; Dessev, T.; Dimitrov, N.; Petkova, N. Effects on the Quality and Health-Enhancing Properties of Industrial Onion Waste Powder on Bread. J. Food Sci. Technol. 2018, 55, 5091–5097. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of Extraction Process of Antioxidant Compounds from Yellow Onion Skin and Their Use in Functional Bread Production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Grape Pomace as Innovative Flour for the Formulation of Functional Muffins: How Particle Size Affects the Nutritional, Textural and Sensory Properties. Foods 2022, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Baldán, Y.; Riveros, M.; Fabani, M.P.; Rodriguez, R. Grape Pomace Powder Valorization: A Novel Ingredient to Improve the Nutritional Quality of Gluten-Free Muffins. Biomass Convers. Biorefin 2021, 1–13. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.A.; Ahmed, A.R. Utilization of Watermelon Rinds and Sharlyn Melon Peels as a Natural Source of Dietary Fiber and Antioxidants in Cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Drabińska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli By-Products Improve the Nutraceutical Potential of Gluten-Free Mini Sponge Cakes. Food Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef]
- Kondyli, E.; Pappa, E.C.; Arapoglou, D.; Metafa, M.; Eliopoulos, C.; Israilides, C. Effect of Fortification with Mushroom Polysaccharide β-Glucan on the Quality of Ovine Soft Spreadable Cheese. Foods 2022, 11, 417. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Fortification of Queso Fresco, Cheddar and Mozzarella Cheese Using Selected Sources of Omega-3 and Some Nonthermal Approaches. Food Chem. 2012, 133, 787–797. [Google Scholar] [CrossRef]
- Solhi, P.; Azadmard-Damirchi, S.; Hesari, J.; Hamishehkar, H. Effect of Fortification with Asparagus Powder on the Qualitative Properties of Processed Cheese. Int. J. Dairy. Technol. 2020, 73, 226–233. [Google Scholar] [CrossRef]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the Glycemic Index of Short Dough Biscuits by Using Apple Pomace as a Functional Ingredient. LWT 2019, 100, 300–305. [Google Scholar] [CrossRef]
- de Toledo, N.M.V.; Nunes, L.P.; da Silva, P.P.M.; Spoto, M.H.F.; Canniatti-Brazaca, S.G. Influence of Pineapple, Apple and Melon By-products on Cookies: Physicochemical and Sensory Aspects. Int. J. Food Sci. Technol. 2017, 52, 1185–1192. [Google Scholar] [CrossRef]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. In Vitro Bioaccessibility and Functional Properties of Polyphenols from Pomegranate Peels and Pomegranate Peels-Enriched Cookies. Food Funct. 2016, 7, 4247–4258. [Google Scholar] [CrossRef] [PubMed]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Benatallah, L.; Zidoune, M.N.; Dziki, D. Pomegranate Seed Powder as a Functional Component of Gluten-free Bread (Physical, Sensorial and Antioxidant Evaluation). Int. J. Food Sci. Technol. 2018, 53, 1906–1913. [Google Scholar] [CrossRef]
- Obafaye, R.O.; Omoba, O.S. Orange Peel Flour: A Potential Source of Antioxidant and Dietary Fiber in Pearl-millet Biscuit. J. Food Biochem. 2018, 42, e12523. [Google Scholar] [CrossRef]
- Ozcan, T.; Yilmaz-Ersan, L.; Akpinar-Bayizit, A.; Delikanli, B. Antioxidant Properties of Probiotic Fermented Milk Supplemented with Chestnut Flour (Castanea Sativa Mill). J. Food Process. Preserv. 2017, 41, e13156. [Google Scholar] [CrossRef]
- Perina, N.P.; Granato, D.; Hirota, C.; Cruz, A.G.; Bogsan, C.S.B.; Oliveira, M.N.d. Effect of Vegetal-Oil Emulsion and Passion Fruit Peel-Powder on Sensory Acceptance of Functional Yogurt. Food Res. Int. 2015, 70, 134–141. [Google Scholar] [CrossRef]
- Demirci, T.; Aktaş, K.; Sözeri, D.; Öztürk, H.İ.; Akın, N. Rice Bran Improve Probiotic Viability in Yoghurt and Provide Added Antioxidative Benefits. J. Funct. Foods 2017, 36, 396–403. [Google Scholar] [CrossRef]
- García, M.L.; Calvo, M.M.; Selgas, M.D. Beef Hamburgers Enriched in Lycopene Using Dry Tomato Peel as an Ingredient. Meat Sci. 2009, 83, 45–49. [Google Scholar] [CrossRef]
- Selani, M.M.; Shirado, G.A.N.; Margiotta, G.B.; Saldaña, E.; Spada, F.P.; Piedade, S.M.S.; Contreras-Castillo, C.J.; Canniatti-Brazaca, S.G. Effects of Pineapple Byproduct and Canola Oil as Fat Replacers on Physicochemical and Sensory Qualities of Low-Fat Beef Burger. Meat Sci. 2016, 112, 69–76. [Google Scholar] [CrossRef]
- Zaini, H.B.M.; Sintang, M.D.B.; Pindi, W. The Roles of Banana Peel Powders to Alter Technological Functionality, Sensory and Nutritional Quality of Chicken Sausage. Food Sci. Nutr. 2020, 8, 5497–5507. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Pezo, L.; Šovljanski, O.; Lević, S.; Nedović, V.; Markov, S.; Tomić, A.; Čanadanović-Brunet, J.; Vulić, J.; Šaponjac, V.T. New Concept of Fortified Yogurt Formulation with Encapsulated Carrot Waste Extract. LWT 2021, 138, 110732. [Google Scholar] [CrossRef]
- Kiros, E.; Seifu, E.; Bultosa, G.; Solomon, W.K. Effect of Carrot Juice and Stabilizer on the Physicochemical and Microbiological Properties of Yoghurt. LWT-Food Sci. Technol. 2016, 69, 191–196. [Google Scholar] [CrossRef]
- Sandoval-Castilla, O.; Lobato-Calleros, C.; Aguirre-Mandujano, E.; Vernon-Carter, E.J. Microstructure and Texture of Yogurt as Influenced by Fat Replacers. Int. Dairy. J. 2004, 14, 151–159. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee Silverskin as Nutraceutical Ingredient in Yogurt: Its Effect on Functional Properties and Its Bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading Players in the Maintenance of Gut Homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef]
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M. Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. Biomed. Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Lutsiv, T.; McGinley, J.N.; Neil-McDonald, E.S.; Weir, T.L.; Foster, M.T.; Thompson, H.J. Relandscaping the Gut Microbiota with a Whole Food: Dose–Response Effects to Common Bean. Foods 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Illiano, P.; Brambilla, R.; Parolini, C. The Mutual Interplay of Gut Microbiota, Diet and Human Disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Maki, J.J.; Klima, C.L.; Sylte, M.J.; Looft, T. The Microbial Pecking Order: Utilization of Intestinal Microbiota for Poultry Health. Microorganisms 2019, 7, 376. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Oriach, C.S.; Robertson, R.C.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Food for Thought: The Role of Nutrition in the Microbiota-Gut–Brain Axis. Clin. Nutr. Exp. 2016, 6, 25–38. [Google Scholar] [CrossRef]
- Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; García-Cayuela, T. Probiotics, Prebiotics, and Synbiotics Added to Dairy Products: Uses and Applications to Manage Type 2 Diabetes. Food Res. Int. 2021, 142, 110208. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. Problems with the Concept of Gut Microbiota Dysbiosis. Microb. Biotechnol. 2020, 13, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Perez, N.B.; Dorsen, C.; Squires, A. Dysbiosis of the Gut Microbiome: A Concept Analysis. J. Holist. Nurs. 2020, 38, 223–232. [Google Scholar] [CrossRef]
- Tiffany, C.R.; Bäumler, A.J. Dysbiosis: From Fiction to Function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G602–G608. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.-M.; Fournier, P.-E.; Raoult, D. Culturing the Human Microbiota and Culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, H.; Chen, P.; Xie, H.; Tao, Y. Demystifying the Manipulation of Host Immunity, Metabolism, and Extraintestinal Tumors by the Gut Microbiome. Signal. Transduct. Target. Ther. 2019, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Fraher, M.H.; O’toole, P.W.; Quigley, E.M.M. Techniques Used to Characterize the Gut Microbiota: A Guide for the Clinician. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 312. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; Hsu, T.; Sirota-Madi, A.; Shafquat, A.; Abu-Ali, G.; Morgan, X.C.; Huttenhower, C. Sequencing and beyond: Integrating Molecular ‘omics’ for Microbial Community Profiling. Nat. Rev. Microbiol. 2015, 13, 360–372. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Westerhuis, J.A. A Beginner’s Guide to Integrating Multi-Omics Data from Microbial Communities. Biochemist 2022, 44, 23–29. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Walton, G.E.; Poveda, C.G.; Silva, S.N.; Amorim, M.; Madureira, A.R.; Pintado, M.E.; Gibson, G.R.; Jauregi, P. Study of in Vitro Digestion of Tenebrio Molitor Flour for Evaluation of Its Impact on the Human Gut Microbiota. J. Funct. Foods 2019, 59. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Oliveira, D.L.; Costa, C.M.; Pintado, M.; Madureira, A.R. Can Supplemented Skim Milk (SKM) Boost Your Gut Health? Fermentation 2022, 8, 126. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Oliveira, D.L.; Saleh, M.A.D.; Pintado, M.; Madureira, A.R. Preservation of Human Gut Microbiota Inoculums for In Vitro Fermentations Studies. Fermentation 2021, 7, 14. [Google Scholar] [CrossRef]
- Scortichini, S.; Boarelli, M.C.; Silvi, S.; Fiorini, D. Development and Validation of a GC-FID Method for the Analysis of Short Chain Fatty Acids in Rat and Human Faeces and in Fermentation Fluids. J. Chromatogr. B 2020, 1143, 121972. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Cardoso, B.B.; Alves, J.I.; Pereira, M.A.; Rodrigues, L.R. In Vitro Fermentation of Raffinose to Unravel Its Potential as Prebiotic Ingredient. LWT 2020, 109322. [Google Scholar]
- Doke, S.K.; Dhawale, S.C. Alternatives to Animal Testing: A Review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Ramiro-Garcia, J.; Koenen, M.E.; Venema, K. To Pool or Not to Pool? Impact of the Use of Individual and Pooled Fecal Samples for in Vitro Fermentation Studies. J. Microbiol. Methods 2014, 107, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of Prebiotics by Human Colonic Microbiota in Vitro and Short Chain Fatty Acids Production: A Critical Review. J. Appl. Microbiol. 2020, 130, 677–687. [Google Scholar]
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.K.; Lee, J.; Yau, Y.F.; Ansell, J.; Theis, S. Developments in Understanding and Applying Prebiotics in Research and Practice—An ISAPP Conference Paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Teixeira, F.; Silva, S.; Madureira, A.R.; Pintado, M. Potential Prebiotic Activity of Tenebrio Molitor Insect Flour Using an Optimized in Vitro Gut Microbiota Model. Food Funct. 2019, 10, 3909–3922. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Zheng, J.; Wen, C.; Ji, C.; Zhang, D.; Chen, Y.; Hou, Z.; Yang, N. Efficacy of Fecal Sampling as a Gut Proxy in the Study of Chicken Gut Microbiota. Front. Microbiol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, X.; Huang, X.; Murff, H.J.; Ness, R.M.; Seidner, D.L.; Sorgen, A.A.; Blakley, I.C.; Yu, C.; Dai, Q. On the Robustness of Inference of Association with the Gut Microbiota in Stool, Rectal Swab and Mucosal Tissue Samples. Sci. Rep. 2021, 11, 14828. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.-R. Gut Flora in Health and Disease. The Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, A.; Butt, M.S.; Afzaal, M.; van Baak, M.; Nadeem, M.T.; Shahid, M.Z. Prebiotics, Gut Microbiota and Metabolic Risks: Unveiling the Relationship. J. Funct. Foods 2015, 17, 189–201. [Google Scholar] [CrossRef]
- Bosch, G.; Wrigglesworth, D.J.; Cone, J.W.; Pellikaan, W.F.; Hendriks, W.H. Effects of Preservation Conditions of Canine Feces on in Vitro Gas Production Kinetics and Fermentation End Products. J. Anim. Sci. 2013, 91, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.M.D.; McMullin, P.; Handel, I.; Hastie, P.M. The Effect of Freezing on the Fermentative Activity of Equine Faecal Inocula for Use in an in Vitro Gas Production Technique. Anim. Feed. Sci. Technol. 2012, 178, 175–182. [Google Scholar] [CrossRef]
- Prates, A.; De Oliveira, J.A.; Abecia, L.; Fondevila, M. Effects of Preservation Procedures of Rumen Inoculum on in Vitro Microbial Diversity and Fermentation. Anim. Feed. Sci. Technol. 2010, 155, 186–193. [Google Scholar] [CrossRef]
- Roupar, D.; Coelho, M.C.; Gonçalves, D.A.; Silva, S.P.; Coelho, E.; Silva, S.; Coimbra, M.A.; Pintado, M.; Teixeira, J.A.; Nobre, C. Evaluation of Microbial-Fructo-Oligosaccharides Metabolism by Human Gut Microbiota Fermentation as Compared to Commercial Inulin-Derived Oligosaccharides. Foods 2022, 11, 954. [Google Scholar] [CrossRef]
- Madureira, A.R.; Campos, D.; Gullon, B.; Marques, C.; Rodríguez-Alcalá, L.M.; Calhau, C.; Alonso, J.L.; Sarmento, B.; Gomes, A.M.; Pintado, M. Fermentation of Bioactive Solid Lipid Nanoparticles by Human Gut Microflora. Food Funct. 2016, 7, 516–529. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in Vitro Batch Fermentation Protocol for Studying the Contribution of Food to Gut Microbiota Composition and Functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Seara, M.; Carvalho, M.J.; de Carvalho, N.M.; Costa, E.M.; Silva, S.; Duarte, M.; Pintado, M.; Oliveira, C.; Madureira, A.R. Production of Sustainable Postbiotics from Sugarcane Straw for Potential Food Applications. Appl. Sci. 2023, 13, 3391. [Google Scholar] [CrossRef]
- Dixit, Y.; Kanojiya, K.; Bhingardeve, N.; Ahire, J.J.; Saroj, D. In Vitro Human Gastrointestinal Tract Simulation Systems: A Panoramic Review. Probiotics Antimicrob. Proteins 2023, 1–18. [Google Scholar] [CrossRef]
- Isenring, J.; Bircher, L.; Geirnaert, A.; Lacroix, C. In Vitro Human Gut Microbiota Fermentation Models: Opportunities, Challenges, and Pitfalls. Microbiome Res. Rep. 2023, 2, 2. [Google Scholar] [CrossRef]
- Madureira, A.R.; Amorim, M.; Gomes, A.M.; Pintado, M.E.; Malcata, F.X. Protective Effect of Whey Cheese Matrix on Probiotic Strains Exposed to Simulated Gastrointestinal Conditions. Food Res. Int. 2011, 44, 465–470. [Google Scholar] [CrossRef]
- Mills, D.J.S.; Tuohy, K.M.; Booth, J.; Buck, M.; Crabbe, M.J.C.; Gibson, G.R.; Ames, J.M. Dietary Glycated Protein Modulates the Colonic Microbiota towards a More Detrimental Composition in Ulcerative Colitis Patients and Non-ulcerative Colitis Subjects. J. Appl. Microbiol. 2008, 105, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Hayes, J.J.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Protection of Candidate Probiotic Lactobacilli by Cheddar Cheese Matrix during Simulated Gastrointestinal Digestion. J. Funct. Foods 2022, 92, 105042. [Google Scholar] [CrossRef]
- Marques, M.C.; Perina, N.P.; Mosquera, E.M.B.; Tomé, T.M.; Lazarini, T.; Mariutti, L.R.B. DHA Bioaccessibility in Infant Formulas and Preschool Children Milks. Food Res. Int. 2021, 149, 110698. [Google Scholar] [CrossRef]
- Faubel, N.; Makran, M.; Cilla, A.; Alegría, A.; Barberá, R.; Garcia-Llatas, G. Bioaccessibility of Plant Sterols in Wholemeal Rye Bread Using the INFOGEST Protocol: Influence of Oral Phase and Enzymes of Lipid Metabolism. J. Agric. Food Chem. 2022, 70, 13223–13232. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Nehir El, S.; Simsek, S. Food Technological Applications for Optimal Nutrition: An Overview of Opportunities for the Food Industry. Compr. Rev. Food Sci. Food Saf. 2012, 11, 2–12. [Google Scholar] [CrossRef]
- Kariyawasam, K.M.G.M.M.; Lee, N.-K.; Paik, H.-D. Fermented Dairy Products as Delivery Vehicles of Novel Probiotic Strains Isolated from Traditional Fermented Asian Foods. J. Food Sci. Technol. 2021, 58, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Ślizewska, K. The Role of Probiotics, Prebiotics and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Gao, J.; Ma, J.; Li, T.; Tan, B.; Huang, X.; Jie, Y. Opportunities of Prebiotics for the Intestinal Health of Monogastric Animals. Anim. Nutr. 2020, 6, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of Food Matrix on the Content and Bioavailability of Flavonoids. Trends Food Sci. Technol. 2021, 117, 15–33. [Google Scholar] [CrossRef]
- Day, L.; Seymour, R.B.; Pitts, K.F.; Konczak, I.; Lundin, L. Incorporation of Functional Ingredients into Foods. Trends Food Sci. Technol. 2009, 20, 388–395. [Google Scholar] [CrossRef]
- Shinn, L.M.; Li, Y.; Mansharamani, A.; Auvil, L.S.; Welge, M.E.; Bushell, C.; Khan, N.A.; Charron, C.S.; Novotny, J.A.; Baer, D.J. Fecal Bacteria as Biomarkers for Predicting Food Intake in Healthy Adults. J. Nutr. 2021, 151, 423–433. [Google Scholar] [CrossRef]
- Weaver, C.M.; Lichtenstein, A.H.; Kris-Etherton, P.M. Perspective: Guidelines Needed for the Conduct of Human Nutrition Randomized Controlled Trials. Adv. Nutr. 2021, 12, 1–3. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Petersen, K.; Barger, K.; Hansen, K.E.; Anderson, C.A.M.; Baer, D.J.; Lampe, J.W.; Rasmussen, H.; Matthan, N.R. Perspective: Design and Conduct of Human Nutrition Randomized Controlled Trials. Adv. Nutr. 2021, 12, 4–20. [Google Scholar] [CrossRef]
- Weaver, C.M.; Fukagawa, N.K.; Liska, D.; Mattes, R.D.; Matuszek, G.; Nieves, J.W.; Shapses, S.A.; Snetselaar, L.G. Perspective: US Documentation and Regulation of Human Nutrition Randomized Controlled Trials. Adv. Nutr. 2021, 12, 21–45. [Google Scholar] [CrossRef]
- Maki, K.C.; Miller, J.W.; McCabe, G.P.; Raman, G.; Kris-Etherton, P.M. Perspective: Laboratory Considerations and Clinical Data Management for Human Nutrition Randomized Controlled Trials: Guidance for Ensuring Quality and Integrity. Adv. Nutr. 2021, 12, 46–58. [Google Scholar] [CrossRef]
- Petersen, K.S.; Kris-Etherton, P.M.; McCabe, G.P.; Raman, G.; Miller, J.W.; Maki, K.C. Perspective: Planning and Conducting Statistical Analyses for Human Nutrition Randomized Controlled Trials: Ensuring Data Quality and Integrity. Adv. Nutr. 2021, 12, 1610–1624. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Hodges, J.K. Designing, Conducting, and Documenting Human Nutrition Plant-Derived Intervention Trials. Front. Nutr. 2021, 8, 782703. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P. Statistical Power and Why It Matters|A Simple Introduction. Available online: https://www.scribbr.com/statistics/statistical-power/ (accessed on 10 October 2022).
- Childs, C.E.; Röytiö, H.; Alhoniemi, E.; Fekete, A.A.; Forssten, S.D.; Hudjec, N.; Lim, Y.N.; Steger, C.J.; Yaqoob, P.; Tuohy, K.M. Xylo-Oligosaccharides Alone or in Synbiotic Combination with Bifidobacterium Animalis Subsp. Lactis Induce Bifidogenesis and Modulate Markers of Immune Function in Healthy Adults: A Double-Blind, Placebo-Controlled, Randomised, Factorial Cross-over Study. Br. J. Nutr. 2014, 111, 1945–1956. [Google Scholar] [CrossRef]
- Oliveira, D.; Nilsson, A. Effects of Dark-Chocolate on Appetite Variables and Glucose Tolerance: A 4 Week Randomised Crossover Intervention in Healthy Middle Aged Subjects. J. Funct. Foods 2017, 37, 390–399. [Google Scholar] [CrossRef]
- Palsson, O.S.; Peery, A.; Seitzberg, D.; Amundsen, I.D.; McConnell, B.; Simrén, M. Human Milk Oligosaccharides Support Normal Bowel Function and Improve Symptoms of Irritable Bowel Syndrome: A Multicenter, Open-Label Trial. Clin. Transl. Gastroenterol. 2020, 11, e00276. [Google Scholar] [CrossRef]





| Product Name | Food Matrix | Food Ingredient Incorporated | Claim |
|---|---|---|---|
| Blevit Plus 8 Cereals and Cookie Maria | Cereal baby porridge | Fructo-oligosaccharides (FOS), Bifidobacterium infantis, Lactobacillus rhamnosus and vitamin complex (e.g., vitamin A, C, and D) | Provides essential micronutrients, encourages normal bone growth and development and maturation of the baby’s digestive and immune systems |
| Website: https://www.blevit.com/producto/blevit-plus-duplo-8-cereales-y-galletas-maria (accessed on 25 May 2023) | |||
| Danone Activia | Yogurt or fermented skimmed milk | Bifidobacterium animalis CNCM I-2494 | Reduces the frequency of intestinal discomfort |
| Website: https://www.danone.pt/marcas/activia (accessed on 25 May 2023) | |||
| John West Energy tuna steak | Tuna | Vitamin B | Reduces tiredness and fatigue |
| John West Immunity tuna steak | Vitamin C | Supports immune system | |
| John West Heart tuna steak | Omega-3 | Supports heart function | |
| Website: https://www.john-west.ie/products/range/nutrient-rich-tuna/ (accessed on 25 May 2023) | |||
| Marigold Vegan Engevita | Yeast flakes | Vitamin B12 | Does not mention the possible outcomes the consumption of it brings, only mention what is included in the food matrices |
| Marigold Super Boost Vegan Engevita | Vitamin D and iron | ||
| Website: http://marigoldhealthfoods.co.uk/products/engevita/ (accessed on 25 May 2023) | |||
| Mimosa Bem Especial | Milk | Calcium and vitamin D | Supports the growth and development of bone mass |
| Website: https://mimosa.com.pt/produtos-lacteos/leite/bem-especial/calcio/ (accessed on 25 May 2023) | |||
| Myvitamins wellness Gut gummies | Mixed berry flavor gummies | Bacillus coagulans and vitamin C | Improves health and well-being, supports immune system, and helps to reduce fatigue |
| Website: https://www.myprotein.com/vitamins/gut-gummies/12552274.html (accessed on 25 May 2023) | |||
| Nestle Bolero | Cereal and fiber-soluble powder | Inulin | Restores energy and maintains the person’s well-being |
| Website: https://saboreiaavida.nestle.pt/produtos/cafe-e-bebidas/bolero-cereais-e-fibra (accessed on 25 May 2023) | |||
| Nestle Kefir Natural | Pasteurized semi-skimmed milk | Kefir grains and yeast | Supports the digestive and immune systems |
| Website: https://saboreiaavida.nestle.pt/produtos/lacteos-e-sobremesas/kefir-natural-150g (accessed on 25 May 2023) | |||
| Sonatural Culturas vivas | Apple juice, carrot ginger juice, or pineapple ginger juice | Inulin, B. coagulans GBI-30 6086, and vitamin C | Improves digestive health, stimulates the immune system, maintains equilibrium of gut microbiota and reduces the activity of harmful bacteria. |
| Website: https://sonatural.pt/collections/shotsprobioticos (accessed on 25 May 2023) | |||
| Vatel Iodized Coarse Sea Salt | Sea salt | Iodine | Supports the normal production of thyroid hormones as well as thyroid function, the nervous system, and cognitive function |
| Website: http://vatel.pt/en/iodized-cooking-sea-salt-1kg/ (accessed on 25 May 2023) | |||
| Yakult Original | Fermented skimmed milk | Lactobacillus casei Shirota | Helps to keep a balanced gut microbiota |
| Yakult Light | L. casei Shirota, vitamin D and E | Helps to keep a balanced gut microbiota, supports the immune system, maintains bone and muscle function, and protects cells from oxidative stress | |
| Website: https://www.yakult.co.uk/products/ (accessed on 25 May 2023) | |||
| Food Matrix | Source (Byproducts) of Bioactive Compounds | Example of Bioactive Compounds Present | Incorporation Tested (%) | Type of Study | References |
|---|---|---|---|---|---|
| Bread | Mango | Carotenoids and polyphenols | 5–25 | Physicochemical studies | [26] |
| Lettuce | Fibers and vitamins | 2–40 | Physicochemical and sensory studies | [27] | |
| Onion | Fibers and polyphenols | 0.1–5 | Physicochemical studies | [28,29] | |
| Cakes | Grape | Fibers and polyphenols | 15–25 | Physicochemical and sensory studies | [30,31] |
| Watermelon and melon | Carotenoids and vitamins | 5–15 | [32] | ||
| Broccoli | Fibers and glucosinolates | 2.5–7.5 | [33] | ||
| Cheese | Mushroom | β-glucans | 0.4 | Physicochemical and sensory studies | [34] |
| Fish | Essential fatty acids | 1 | Physicochemical and microbiological studies | [35] | |
| Asparagus | Anthocyanins and fibers | 0.5 to 1.5 | Physicochemical and sensory studies | [36] | |
| Cookies | Apple | Fibers and polyphenols | 10–20 | Physicochemical, sensory, and /or in vitro studies | [37,38] |
| Pomegranate | 2.5–10 | [39,40] | |||
| Orange | 5–20 | Physicochemical and sensory studies | [41] | ||
| Fermented milk | Chestnut | Minerals and vitamins | 2 | Physicochemical and microbiological studies | [42] |
| Passion fruit | Fibers and vitamins | 1 | Physicochemical, microbiological, and sensory studies | [43] | |
| Rice | Fibers and minerals | 1–3 | [44] | ||
| Meat | Tomato | Carotenoids and fibers | 1.5–6 | Physicochemical and sensory studies | [45] |
| Pineapple | Fibers and vitamins | 1.5 | [46] | ||
| Banana | Fibers and minerals | 2–6 | [47] | ||
| Yogurt | Carrots | Carotenoids and vitamins | 2.5–20 | Physicochemical, microbiological, and/or in vitro studies | [48,49] |
| Dairy | Proteins and peptides | 0.33–1 | Physicochemical studies | [50] | |
| Coffee | Fibers and polyphenols | 2–6 | Physicochemical, microbiological, and in vitro studies | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, N.M.; Oliveira, D.L.; Costa, C.M.; Pintado, M.E.; Madureira, A.R. Strategies to Assess the Impact of Sustainable Functional Food Ingredients on Gut Microbiota. Foods 2023, 12, 2209. https://doi.org/10.3390/foods12112209
de Carvalho NM, Oliveira DL, Costa CM, Pintado ME, Madureira AR. Strategies to Assess the Impact of Sustainable Functional Food Ingredients on Gut Microbiota. Foods. 2023; 12(11):2209. https://doi.org/10.3390/foods12112209
Chicago/Turabian Stylede Carvalho, Nelson Mota, Diana Luazi Oliveira, Célia Maria Costa, Manuela Estevez Pintado, and Ana Raquel Madureira. 2023. "Strategies to Assess the Impact of Sustainable Functional Food Ingredients on Gut Microbiota" Foods 12, no. 11: 2209. https://doi.org/10.3390/foods12112209
APA Stylede Carvalho, N. M., Oliveira, D. L., Costa, C. M., Pintado, M. E., & Madureira, A. R. (2023). Strategies to Assess the Impact of Sustainable Functional Food Ingredients on Gut Microbiota. Foods, 12(11), 2209. https://doi.org/10.3390/foods12112209