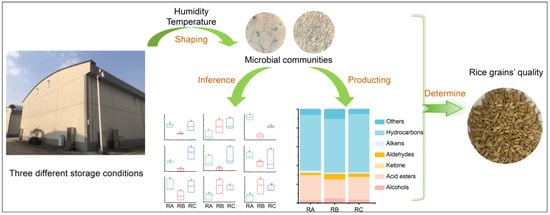
Deciphering the Microbiological Mechanisms Underlying the Impact of Different Storage Conditions on Rice Grain Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. DNA Extraction, Sequencing, and Bioinformatics Analysis
2.3. Determination of Physical and Chemical Indicators
2.3.1. Detection of Ochratoxin A and Aflatoxin B1 in Rice Grain Samples
2.3.2. Moisture Content Detection
2.3.3. Starch Content Detection
2.3.4. The Content of Soluble Proteins Detection
2.3.5. Fatty Acid Value Detection
2.3.6. Volatile Component Detection
2.4. Statistical Analysis
3. Results and Discussion
3.1. Determination of Physicochemical Indices
3.2. Comparison of Volatile Components of Rice Grains Stored under Different Conditions
3.3. Comparison of Mycotoxins of Rice Grains Stored under Different Conditions
3.4. Determination of Microbial Community Structure
3.5. Correlations between Physicochemical Indices and the Microbial Community
3.6. Relationships between Environmental Factors and Microbial Community
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| N0 | Compound Name | Storage Conditions (Mean ± SD) | ||
|---|---|---|---|---|
| RA | RB | RC | ||
| 1 | 2-Methyldodecane | ND | ND | 1.16 ± 1.01 |
| 2 | 4,8-Dimethyltridecane | ND | ND | 5.32 ± 0.1 |
| 3 | 11-(1-Ethylpropyl)-undecane | ND | ND | 2.79 ± 0.16 |
| 4 | 2,6,10-Trimethyldodecane | 7.94 ± 1.74 b | 8.3 ± 1.93 b | 22.25 ± 1.96 a |
| 5 | Ethyl 9-Hexadecenoate | ND | ND | 1.58 ± 0.47 a |
| 6 | 2-Isopropyl-5-Methyl-1-heptanol | ND | ND | 5.54 ± 1.68 a |
| 7 | 3-Methylundecane | ND | ND | 3.96 ± 1.47 a |
| 8 | Sulfurous acid, 2-Pentyl Undecyl ester | ND | 9.3 ± 0.21 a | ND |
| 9 | 2,5-Dimethyltridecane | ND | ND | 3.5 ± 1.53 a |
| 10 | 10-Methyleicosane | 5.32 ± 0.10 a | ND | ND |
| 11 | 1-Dodecene | 3.39 ± 0.24 a | ND | ND |
| 12 | 2,6,10,14-Tetramethylhexadecane | 5.13 ± 0.98 a | ND | ND |
| 13 | Tetradecane | 14.71 ± 1.09 a | 6.84 ± 0.54 b | 7.42 ± 1.26 b |
| 14 | Sulfurous acid, dodecyl pentyl ester | ND | 9.3 ± 0.21 a | ND |
| 15 | Heneicosane | ND | 5.62 ± 0.19 a | ND |
| 16 | 2-Pentylfuran | ND | 10.57 ± 0.44 a | ND |
| 17 | 2-Methyltridecane | 9.97 ± 0.86 a | 12.58 ± 2.74 a | 11.77 ± 1.35 a |
| 18 | 3,5-Dimethyldodecane | ND | 2.56 ± RB0.37 a | ND |
| 19 | 4-Methylhexadecane | ND | 2.03 ± 0.31 a | ND |
| 20 | Octadecane | ND | 4.72 ± 0.72 a | ND |
| 21 | 4-Ethyldecane | ND | 2.92 ± 0.45 a | ND |
| 22 | Tridecane | 5.78 ± 1.13 a | ND | ND |
| 23 | 5,7-Dimethylundecane | ND | 5.32 ± 4.83 a | ND |
| 24 | 1,2-Benzenedicarboxylic acid, butyl ester | ND | 4 ± 0.66 | 3.2 ± 0.3 |
| 25 | 1,3-Dichlorobenzene | 19.07 ± 17.9 b | 50.8 ± 3.13 a | ND |
| 26 | 2-Hexyl-1-decanol | ND | 5.59 ± 0.52 a | 1.81 ± 0.13 b |
| 27 | 4,6-Dimethyldodecane | ND | ND | 7.06 ± 4.11 a |
| 28 | 5-Methyloctadecane | ND | 9.17 ± 3.04 a | 10.9 ± 3.42 a |
| 29 | 2,6,11,15-Tetramethylhexadecane | ND | ND | 4.01 ± 2.4 a |
| 30 | Heptadecane | ND | 11.77 ± 1.21 a | 15.09 ± 7.07 a |
| 31 | 4-Methylundecane | ND | 3.02 ± 0.92 a | 2.47 ± 0.27 a |
| 32 | Hexadecane | ND | 11.77 ± 1.21 a | 15.09 ± 7.07 a |
| 33 | 2-Butyl-1-octanol | ND | 4.85 ± 2.02 a | ND |
| 34 | 2,6,10,15-Tetramethylheptadecane | 5.37 ± 0.8 a | ND | ND |
| 35 | 2-Methylheptadecane | ND | 1.67 ± 0.95 a | ND |




References
- Peng, S.; Tang, Q.; Zou, Y. Current Status and Challenges of Rice Production in China. Plant Prod. Sci. 2009, 12, 3–8. [Google Scholar] [CrossRef]
- Du, J.; Lin, Y.; Gao, Y.; Tian, Y.; Zhang, J.; Fang, G. Nutritional Changes and Early Warning of Moldy Rice under Different Relative Humidity and Storage Temperature. Foods 2022, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Ranmeechai, N.; Photchanachai, S. Effect of Modified Atmosphere Packaging on the Quality of Germinated Parboiled Brown Rice. Food Sci. Biotechnol. 2017, 26, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Martín Castaño, S.; Medina, A.; Magan, N. Comparison of Dry Matter Losses and Aflatoxin B1 Contamination of Paddy and Brown Rice Stored Naturally or after Inoculation with Aspergillus Flavus at Different Environmental Conditions. J. Stored Prod. Res. 2017, 73, 47–53. [Google Scholar] [CrossRef]
- Schmidt, M.; Horstmann, S.; De Colli, L.; Danaher, M.; Speer, K.; Zannini, E.; Arendt, E.K. Impact of Fungal Contamination of Wheat on Grain Quality Criteria. J. Cereal Sci. 2016, 69, 95–103. [Google Scholar] [CrossRef]
- Choi, S.; Jun, H.; Bang, J.; Chung, S.-H.; Kim, Y.; Kim, B.; Kim, H.; Beuchat, L.R.; Ryu, J.-H. Behaviour of Aspergillus Flavus and Fusarium Graminearum on Rice as Affected by Degree of Milling, Temperature, and Relative Humidity during Storage. Food Microbiol. 2015, 46, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Kim, K.D. Effect of Temperature and Relative Humidity on Growth of Aspergillus and Penicillium spp. and Biocontrol Activity of Pseudomonas Protegens AS15 against Aflatoxigenic Aspergillus Flavus in Stored Rice Grains. Mycobiology 2018, 46, 287–295. [Google Scholar] [CrossRef]
- Olstorpe, M.; Schnürer, J.; Passoth, V. Microbial Changes during Storage of Moist Crimped Cereal Barley Grain under Swedish Farm Conditions. Anim. Feed. Sci. Technol. 2010, 156, 37–46. [Google Scholar] [CrossRef]
- Park, C.-E.; Kim, Y.-S.; Park, K.-J.; Kim, B.-K. Changes in Physicochemical Characteristics of Rice during Storage at Different Temperatures. J. Stored Prod. Res. 2012, 48, 25–29. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of Temperature and Water Activity on Deleterious Fungi and Mycotoxin Production during Grain Storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Mohammadi Shad, Z.; Atungulu, G.G. Post-Harvest Kernel Discoloration and Fungi Activity in Long-Grain Hybrid, Pureline and Medium-Grain Rice Cultivars as Influenced by Storage Environment and Antifungal Treatment. J. Stored Prod. Res. 2019, 81, 91–99. [Google Scholar] [CrossRef]
- García, Y.; Santos, C.; Cajarville, C.; Suárez, G.; Bettucci, L. How Do Time, Tannin, and Moisture Content Influence Toxicogenic Fungal Populations during the Storage of Sorghum Grains? J. Food Prot. 2022, 85, 778–785. [Google Scholar] [CrossRef]
- Bahar, A.; Lichter, A. Effect of Controlled Atmosphere on the Storage Potential of Ottomanit Fig Fruit. Sci. Hortic. 2018, 227, 196–201. [Google Scholar] [CrossRef]
- Dong, X.; He, Y.; Yuan, C.; Cheng, X.; Li, G.; Shan, Y.; Zhu, X. Controlled Atmosphere Improves the Quality, Antioxidant Activity and Phenolic Content of Yellow Peach during the Shelf Life. Antioxidants 2022, 11, 2278. [Google Scholar] [CrossRef] [PubMed]
- Mylona, K.; Magan, N. Fusarium Langsethiae: Storage Environment Influences Dry Matter Losses and T2 and HT-2 Toxin Contamination of Oats. J. Stored Prod. Res. 2011, 47, 321–327. [Google Scholar] [CrossRef]
- He, P.; Hassan, M.M.; Tang, F.; Jiang, H.; Chen, M.; Liu, R.; Lin, H.; Chen, Q. Total Fungi Counts and Metabolic Dynamics of Volatile Organic Compounds in Paddy Contaminated by Aspergillus Niger During Storage Employing Gas Chromatography-Ion Mobility Spectrometry. Food Anal. Methods 2022, 15, 1638–1651. [Google Scholar] [CrossRef]
- Chai, Q.; Li, Y.; Li, X.; Wu, W.; Peng, H.; Jia, R.; Sun, Q. Assessment of Variation in Paddy Microbial Communities under Different Storage Temperatures and Relative Humidity by Illumina Sequencing Analysis. Food Res. Int. 2019, 126, 108581. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, F.; Fang, Y.; Li, P.; Xia, J.; Sun, L.; Zou, Y.; Shen, F.; Hu, Q. Interactions among Fungal Community, Fusarium Mycotoxins, and Components of Harvested Wheat under Simulated Storage Conditions. J. Agric. Food Chem. 2019, 67, 8411–8418. [Google Scholar] [CrossRef]
- Li, W.; Cui, J.; Li, J.; Guo, J.; Huang, T.; Zhang, J.; Hu, H.; Liu, X. Analysis of the Fungi Community Variation during Rice Storage through High Throughput Sequencing. Processes 2022, 10, 754. [Google Scholar] [CrossRef]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- He, Q.-H.; Xu, Y.; Wang, D.; Kang, M.; Huang, Z.-B.; Li, Y.-P. Simultaneous Multiresidue Determination of Mycotoxins in Cereal Samples by Polyvinylidene Fluoride Membrane Based Dot Immunoassay. Food Chem. 2012, 134, 507–512. [Google Scholar] [CrossRef]
- Klomklao, P.; Kuntinugunetanon, S.; Wongkokua, W. Moisture Content Measurement in Paddy. J. Phys. Conf. Ser. 2017, 901, 012068. [Google Scholar] [CrossRef]
- Pereira, J.C.A.; da Silva, W.P.; da Silva, R.C.; e Silva, C.M.D.P.S.; Gomes, J.P. Use of Empirical and Diffusion Models in the Description of the Process of Water Absorption by Rice. Eng. Comput. 2022, 39, 1556–1574. [Google Scholar] [CrossRef]
- Fang, Y.; Catron, B.; Zhang, Y.; Zhao, L.; Caruso, J.A.; Hu, Q. Distribution and in Vitro Availability of Selenium in Selenium-Containing Storage Protein from Selenium-Enriched Rice Utilizing Optimized Extraction. J. Agric. Food Chem. 2010, 58, 9731–9738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Z.; Khan, M.U.; Gao, X.; Yu, M.; Gao, H.; Li, Y.; Zhang, H.; Dasanayaka, B.P.; Lin, H. Extraction of Total Wheat (Triticum aestivum) Protein Fractions and Cross-Reactivity of Wheat Allergens with Other Cereals. Food Chem. 2021, 347, 129064. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Liu, Y.; Jia, M.; Wang, S.; Kang, X.; Sun, H.; Strappe, P.; Zhou, Z. Moisture Content Is a Key Factor Responsible for Inducing Rice Yellowing. J. Cereal Sci. 2020, 94, 102988. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, W.; Li, S.; Wang, Y.; Ma, Y.; Meng, X.; Zhang, Y. Comprehensive Evaluation of Paddy Quality by Different Drying Methods, Based on Gray Relational Analysis. Agriculture 2022, 12, 1857. [Google Scholar] [CrossRef]
- Carvalho, M.O.; Fradinho, P.; Martins, M.J.; Magro, A.; Raymundo, A.; de Sousa, I. Paddy Rice Stored under Hermetic Conditions: The Effect of Relative Humidity, Temperature and Storage Time in Suppressing Sitophilus Zeamais and Impact on Rice Quality. J. Stored Prod. Res. 2019, 80, 21–27. [Google Scholar] [CrossRef]
- Ziegler, V.; Paraginski, R.T.; Ferreira, C.D. Grain Storage Systems and Effects of Moisture, Temperature and Time on Grain Quality—A Review. J. Stored Prod. Res. 2021, 91, 101770. [Google Scholar] [CrossRef]
- Hu, X.; Fang, C.; Zhang, W.; Lu, L.; Guo, Z.; Li, S.; Chen, M. Change in Volatiles, Soluble Sugars and Fatty Acids of Glutinous Rice, Japonica Rice and Indica Rice during Storage. LWT 2023, 174, 114416. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, T.; He, P.; Chen, Q. Quantitative Analysis of Fatty Acid Value during Rice Storage Based on Olfactory Visualization Sensor Technology. Sens. Actuators B Chem. 2020, 309, 127816. [Google Scholar] [CrossRef]
- Gobbi, E.; Falasconi, M.; Torelli, E.; Sberveglieri, G. Electronic Nose Predicts High and Low Fumonisin Contamination in Maize Cultures. Food Res. Int. 2011, 44, 992–999. [Google Scholar] [CrossRef]
- Ocan, D.; Rongrong, Z.; Odoch, M.; Nuwamanya, E.; Ibanda, A.P.; Odong, T.L.; Lamo, J.; Fitzgerald, A.M.; Daygon, V.D.; Rubaihayo, P.R. Volatile Organic Compound Based Markers for the Aroma Trait of Rice Grain. J. Agric. Sci. 2020, 12, 92. [Google Scholar] [CrossRef]
- Polizzi, V.; Adams, A.; De Saeger, S.; Van Peteghem, C.; Moretti, A.; De Kimpe, N. Influence of Various Growth Parameters on Fungal Growth and Volatile Metabolite Production by Indoor Molds. Sci. Total Environ. 2012, 414, 277–286. [Google Scholar] [CrossRef]
- Chen, T.; Liu, C.; Meng, L.; Lu, D.; Chen, B.; Cheng, Q. Early Warning of Rice Mildew Based on Gas Chromatography-Ion Mobility Spectrometry Technology and Chemometrics. Food Meas. 2021, 15, 1939–1948. [Google Scholar] [CrossRef]
- Marín, S.; Vinaixa, M.; Brezmes, J.; Llobet, E.; Vilanova, X.; Correig, X.; Ramos, A.J.; Sanchis, V. Use of a MS-Electronic Nose for Prediction of Early Fungal Spoilage of Bakery Products. Int. J. Food Microbiol. 2007, 114, 10–16. [Google Scholar] [CrossRef]
- Le Nguyen Doan, D.; Nguyen, Q.C.; Marini, F.; Biancolillo, A. Authentication of Rice (Oryza sativa L.) Using Near Infrared Spectroscopy Combined with Different Chemometric Classification Strategies. Appl. Sci. 2021, 11, 362. [Google Scholar] [CrossRef]
- Al Husnain, L.; AlKahtani, M.D.F.; Ameen, F. Molecular Detection of Mycobiota and the Associated Mycotoxins in Rice Grains Imported into Saudi Arabia. J. Saudi Soc. Agric. Sci. 2021, 20, 25–30. [Google Scholar] [CrossRef]
- Hanf, B.; Krüger, T.; Mattern, D.; Kniemeyer, O.; Brakhage, A.A. Adaption of the Filamentous Fungus Aspergillus Nidulans to Low Temperature Stress. Cryobiology 2015, 71, 538. [Google Scholar] [CrossRef]
- Chiotta, M.L.; Fumero, M.V.; Cendoya, E.; Palazzini, J.M.; Alaniz-Zanon, M.S.; Ramirez, M.L.; Chulze, S.N. Toxigenic Fungal Species and Natural Occurrence of Mycotoxins in Crops Harvested in Argentina. Rev. Argent. Microbiol. 2020, 52, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.; Ghazali, F.M.; Jinap, S.; Ghazali, H.M.; Radu, S. Modeling Growth Rate and Assessing Aflatoxins Production by Aspergillus flavus as a Function of Water Activity and Temperature on Polished and Brown Rice. J. Food Sci. 2013, 78, M56–M63. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; Devliehgere, F.; De Meulenaer, B.; Debevere, J. Effect of Water Activity and Temperature on Growth and the Relationship between Fumonisin Production and the Radial Growth of Fusarium Verticillioides and Fusarium Proliferatum on Corn. J. Food Prot. 2005, 68, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Q.; Dong, W.; Liu, Y.; Wei, S.; Zuo, M. FEDformer-Based Paddy Quality Assessment Model Affected by Toxin Change in Different Storage Environments. Foods 2023, 12, 1681. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; Devlieghere, F.; Geeraerd, A.; Demeulenaer, B.; Vanimpe, J.; Debevere, J. Modelling of the Individual and Combined Effects of Water Activity and Temperature on the Radial Growth of Aspergillus Flavus and A. Parasiticus on Corn. Food Microbiol. 2007, 24, 517–529. [Google Scholar] [CrossRef]
- He, X.; Liu, H.; Lv, C.; Wang, F.; Zhao, C.; Tao, R.; Li, J.; Liu, Z.; Du, L. Analysis of Rice Microbial Communities under Different Storage Conditions Using Culture-Dependent and -Independent Techniques. Qual. Assur. Saf. Crops Foods 2022, 14, 1–11. [Google Scholar] [CrossRef]
- Tohno, M.; Kobayashi, H.; Tajima, K.; Uegaki, R. Strain-Dependent Effects of Inoculation of Lactobacillus plantarum Subsp. Plantarum on Fermentation Quality of Paddy Rice (Oryza sativa L. Subsp. Japonica) Silage. FEMS Microbiol. Lett. 2012, 337, 112–119. [Google Scholar] [CrossRef]
- Gannibal, P.B.; Orina, A.S.; Kononenko, G.P.; Burkin, A.A. Distinction of Alternaria Sect. Pseudoalternaria Strains among Other Alternaria Fungi from Cereals. J. Fungi 2022, 8, 423. [Google Scholar] [CrossRef]
- Doni, F.; Ishak, M.N.; Suhaimi, N.S.M.; Syaputri, Y.; Han, L.; Mohamed, Z.; Mispan, M.S. Leaf Blight Disease of Rice Caused by Pantoea: Profile of an Increasingly Damaging Disease in Rice. Trop. Plant Pathol. 2022, 48, 1–10. [Google Scholar] [CrossRef]
- Kruse, J.; Doehlemann, G.; Kemen, E.; Thines, M. Asexual and Sexual Morphs of Moesziomyces Revisited. IMA Fungus 2017, 8, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.U.V.; Bento, D.A.V.; de Souza, A.P.; de Souza, C.L. QTL Mapping for Reaction to Phaeosphaeria Leaf Spot in a Tropical Maize Population. Theor. Appl. Genet 2009, 119, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhou, X.; Tian, L.; Zhang, H.; Cai, L.; Tang, F. Distribution of Mycotoxin-Producing Fungi across Major Rice Production Areas of China. Food Control 2022, 134, 108572. [Google Scholar] [CrossRef]
- Yuan, Q.-S.; Yang, P.; Wu, A.-B.; Zuo, D.-Y.; He, W.-J.; Guo, M.-W.; Huang, T.; Li, H.-P.; Liao, Y.-C. Variation in the Microbiome, Trichothecenes, and Aflatoxins in Stored Wheat Grains in Wuhan, China. Toxins 2018, 10, 171. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Hu, T.; Sun, C.; Wu, W. Experimental Study on the Status of Maize Mycotoxin Production in Farmers’ Grain Storage Silos in Northeastern China. Toxins 2021, 13, 741. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Bala, B.K.; Arshad, F.M.; Haque, M.A.; Hossain, M.A. Food Security through Increasing Technical Efficiency and Reducing Postharvest Losses of Rice Production Systems in Bangladesh. Food Sec. 2016, 8, 361–374. [Google Scholar] [CrossRef]
- Romanenko, L.A.; Uchino, M.; Kalinovskaya, N.I.; Mikhailov, V.V. Isolation, Phylogenetic Analysis and Screening of Marine Mollusc-Associated Bacteria for Antimicrobial, Hemolytic and Surface Activities. Microbiol. Res. 2008, 163, 633–644. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Rocha, A.; Sulyok, M.; Krska, R.; Mallmann, C.A. Natural Mycotoxin Contamination of Maize (Zea mays L.) in the South Region of Brazil. Food Control 2017, 73, 127–132. [Google Scholar] [CrossRef]






| Storage Environment | Samples | Granary Temperature (℃) | Air Temperature (℃) | Granary Humidity (%) | Air Humidity (%) | Grain Depot |
|---|---|---|---|---|---|---|
| RA | RA.1 | 13.6 | 14 | 50.9 | 82 | Shaoxing |
| RA | RA.2 | 13.5 | 8 | 59.75 | 82 | Shaoxing |
| RA | RA.3 | 12.4 | 8 | 68.9 | 82 | Shaoxing |
| RA | RA.4 | 12.8 | 8 | 61.2 | 82 | Ningbo |
| RA | RA.5 | 12.8 | 8 | 61.2 | 82 | Ningbo |
| RA | RA.6 | 15.4 | 8 | 68.9 | 82 | Ningbo |
| RB | RB.1 | 12 | 8 | 70.7 | 82 | Quzhou |
| RB | RB.2 | 11.5 | 8 | 71 | 82 | Quzhou |
| RB | RB.3 | 11.4 | 8 | 66.8 | 82 | Quzhou |
| RC | RC.1 | 13.7 | 8 | 64.9 | 82 | Hangzhou |
| RC | RC.2 | 13.5 | 8 | 63.5 | 82 | Hangzhou |
| RC | RC.3 | 13.5 | 15 | 63.5 | 82 | Hangzhou |
| RC | RC.4 | 15 | 8 | 68.4 | 82 | Hangzhou |
| N0 | Compound Name | Storage Conditions (Mean ± SD) | ||
|---|---|---|---|---|
| RA | RB | RC | ||
| Alcohols | ||||
| 1 | 2-Ethyl-1-hexanol | 15.78 ± 1.41 a | 22.04 ± 4.61 a | 15.52 ± 0.62 a |
| 2 | 2-Hexyl-1-decanol | ND | 5.59 ± 0.52 a | 1.81 ± 0.13 b |
| 3 | 2-Butyl-1-octanol | ND | 4.85 ± 2.02 a | ND |
| 4 | 2-Isopropyl-5-Methyl-1-heptanol | ND | ND | 5.54 ± 1.68 a |
| Acid esters | ||||
| 1 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 7.20 ± 4.76 a | 4.03 ± 1.19 a | 3.61 ± 0.46 a |
| 2 | Ethyl dodecanoate | 6.6 ± 0.06 a | 6.41 ± 1.71 a | 6.75 ± 1.56 a |
| 3 | Ethyl heptanoate | 18.40 ± 3.15 a | 16.87 ± 6.78 a | 16.02 ± 4.06 a |
| 4 | Ethyl hexadecanoate | 5.02 ± 2.18 a | 23.40 ± 7.52 a | 18.20 ± 12.49 a |
| 5 | Ethyl nonanoate | 48.72 ± 2.50 a | 30.17 ± 5.67 a | 41.68 ± 13.50 a |
| 6 | Ethyl octanoate | 22.65 ± 7.14 a | 16.61 ± 5.63 a | 21.05 ± 5.04 a |
| 7 | Ethyl myristate | 6.78 ± 0.37 a | 5.67 ± 2.56 a | 14.74 ± 12.67 a |
| 8 | 1,2-Benzenedicarboxylic acid, butyl ester | ND | 3.81 ± 0.81 a | 3.06 ± 0.25 a |
| 9 | Sulfurous acid, dodecyl pentyl ester | ND | 9.3 ± 0.21 a | ND |
| 10 | Ethyl 9-hexadecenoate | ND | ND | 1.58 ± 0.47 a |
| 11 | Sulfurous acid, 2-pentyl undecyl ester | 10.98 ± 0.71 a | ND | ND |
| Ketone | ||||
| 1 | 6,10,14-Trimethyl-2-pentadecanone | 8.51 ± 2.72 a | 6.17 ± 1.33 a | 8.67 ± 2.81 a |
| Aldehydes | ||||
| 1 | Nonanal | 17.55 ± 5.58 a | 36.15 ± 10.09 a | 20.42 ± 7.65 a |
| Alkenes | ||||
| 1 | 1-Dodecene | 3.39 ± 0.24 a | ND | ND |
| 2 | 4,6,8-Trimethyl- 1-nonene -1-Nonene, | 3.57 ± 0.59 a | 3.99 ± 1.02 a | ND |
| 3 | 1-Tetradecene | 4.28 ± 0.50 a | 4.78 ± 0.35 a | 4.45 ± 0.05 a |
| Hydrocarbons | ||||
| 1 | 2,3,5,8-Tetramethyldecane- | 6.80 ± 7.34 a | ND | ND |
| 2 | Dodecane | 11.45 ± 1.19 a | 15.04 ± 3.12 a | 13.02 ± 1.47 a |
| 3 | 2,6,10-Trimethyldodecane | 7.94 ± 1.74 b | 8.3 ± 1.93 b | 22.25 ± 1.96 a |
| 4 | 2,6,11-Trimethyldodecane | 51.61 ± 6.01 a | 54.22 ± 11.98 a | 45.62 ± 18.80 a |
| 5 | 4-Cyclohexyldodecane Dodecane | 7.26 ± 1.00 a | 7.32 ± 0.61 a | 7.39 ± 0.51 a |
| 6 | 5-Methyldodecane | 6.18 ± 0.25 a | 5.80 ± 0.03 a | ND |
| 7 | Eicosane | 29.75 ± 3.49 a | 51.58 ± 19.06 a | 35.92 ± 18.65 a |
| 8 | 10-Methyl- eicosane Eicosane | 5.32 ± 0.10 a | ND | ND |
| 9 | Heptadecane | ND | 11.77 ± 1.21 a | 15.09 ± 7.07 a |
| 10 | 2,6,10,15-Tetramethylheptadecane | 5.37 ± 0.8 a | ND | ND |
| 11 | 2,6,10,14-Tetramethylhexadecane | 5.13 ± 0.98 a | ND | ND |
| 12 | Nonadecane | 22.81 ± 12.74 a | 22.35 ± 6.78 a | 20.32 ± 7.82 a |
| 13 | Pentadecane | 15.38 ± 13.30 a | 17.14 ± 3.74 a | 21.35 ± 6.26 a |
| 14 | 2-Methylpentadecane | 2.64 ± 0.10 a | 2.51 ± 0.35 a | 2.82 ± 0.22 a |
| 15 | 3-Methylpentadecane | 7.82 ± 0.07 a | 8.08 ± 0.28 a | 7.90 ± 0.75 a |
| 16 | Tetradecane | 14.71 ± 1.09 a | 6.84 ± 0.54 b | 7.42 ± 1.26 b |
| 17 | Tridecane | 5.78 ± 1.13 a | ND | ND |
| 18 | 2-Methyltridecane | 9.97 ± 0.86 a | 12.58 ± 2.74 a | 11.77 ± 1.35 a |
| 19 | 3-Methyltridecane | 10.12 ± 1.15 a | 12.58 ± 2.74 a | 11.77 ± 1.35 a |
| 20 | 4-Methyltridecane | 1.96 ± 0.18 a | 2.60 ± 0.04 a | 2.54 ± 0.27 a |
| 21 | 2,4-Dimethylundecane | 17.76 ± 0.92 a | 16.91 ± 1.87 a | 14.85 ± 5.03 a |
| 22 | 2,6-Dimethylundecane | 8.58 ± 0.89 a | 11.63 ± 4.03 a | 10.60 ± 3.33 a |
| 23 | 3-Methyleneundecane | 1.85 ± 0.40 b | 6.11 ± 2.24 a | 2.13 ± 0.44 b |
| 24 | 4,8-Dimethylundecane | 5.12 ± 1.52 a | 7.14 ± 3.80 a | 6.75 ± 1.15 a |
| 25 | 3,5-Dimethyldodecane | ND | 2.56 ± 0.37 a | ND |
| 26 | Cyclotridecane | ND | 3.80 ± 0.03 a | ND |
| 27 | 4-Ethyldecane | ND | 2.92 ± 0.45 a | ND |
| 28 | Heneicosane | ND | 5.62 ± 0.19 a | ND |
| 29 | 2-Methylheptadecane | ND | 1.67 ± 0.95 a | ND |
| 30 | Hexadecane | ND | 11.77 ± 1.21 a | 15.09 ± 7.07 a |
| 31 | 4-Methylhexadecane | ND | 2.03 ± 0.31 a | ND |
| 32 | 4,5-Dimethylnonane | ND | 10.69 ± 4.66 a | 8.61 ± 3.84 a |
| 33 | Octadecane | ND | 4.72 ± 0.72 a | ND |
| 34 | 5-Methyloctadecane | ND | 9.17 ± 3.04 a | 10.90 ± 3.42 a |
| 35 | 4-Methylundecane | ND | 3.02 ± 0.92 a | 2.47 ± 0.27 a |
| 36 | 5,7-Dimethylundecane | ND | 5.32 ± 4.83 a | ND |
| 37 | 2-Methyldodecane | ND | ND | 1.16 ± 1.01 a |
| 38 | 4,6-Dimethyldodecane | ND | ND | 7.06 ± 4.11 a |
| 39 | 5-Methyldodecane | ND | ND | 5.61 ± 0.03 a |
| 40 | 11-(1-Ethylpropyl)-heneicosane | ND | ND | 2.79 ± 0.16 a |
| 41 | 2,6,11,15-Tetramethylhexadecane Hexadecane | ND | ND | 4.01 ± 2.4 a |
| 42 | 2,5-Dimethyltridecane | ND | ND | 3.5 ± 1.53 a |
| 43 | 4,8-Dimethyltridecane | ND | ND | 5.32 ± 0.1 a |
| 44 | 3-Methyleneundecane | ND | ND | 3.96 ± 1.47 a |
| Others | ||||
| 1 | 1,3-Dichlorobenzene | 19.07 ± 17.9 b | 50.8 ± 3.13 a | ND |
| 2 | 2,4-Di-tert-butylphenol | 13.53 ± 1.20 a | 14.22 ± 0.96 a | ND |
| 3 | 2-Pentylfuran | ND | 10.57 ± 0.44 a | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Wu, F.; Hu, H.; Guo, J.; Wu, C.; Wang, P.; Ling, J.; Cui, Y.; Ye, J.; Fang, G.; et al. Deciphering the Microbiological Mechanisms Underlying the Impact of Different Storage Conditions on Rice Grain Quality. Foods 2024, 13, 266. https://doi.org/10.3390/foods13020266
Qiu Z, Wu F, Hu H, Guo J, Wu C, Wang P, Ling J, Cui Y, Ye J, Fang G, et al. Deciphering the Microbiological Mechanisms Underlying the Impact of Different Storage Conditions on Rice Grain Quality. Foods. 2024; 13(2):266. https://doi.org/10.3390/foods13020266
Chicago/Turabian StyleQiu, Zhuzhu, Fenghua Wu, Hao Hu, Jian Guo, Changling Wu, Peng Wang, Jiangang Ling, Yan Cui, Jing Ye, Guanyu Fang, and et al. 2024. "Deciphering the Microbiological Mechanisms Underlying the Impact of Different Storage Conditions on Rice Grain Quality" Foods 13, no. 2: 266. https://doi.org/10.3390/foods13020266
APA StyleQiu, Z., Wu, F., Hu, H., Guo, J., Wu, C., Wang, P., Ling, J., Cui, Y., Ye, J., Fang, G., & Liu, X. (2024). Deciphering the Microbiological Mechanisms Underlying the Impact of Different Storage Conditions on Rice Grain Quality. Foods, 13(2), 266. https://doi.org/10.3390/foods13020266