Recent Highlights in Sustainable Bio-Based Edible Films and Coatings for Fruit and Vegetable Applications
Abstract
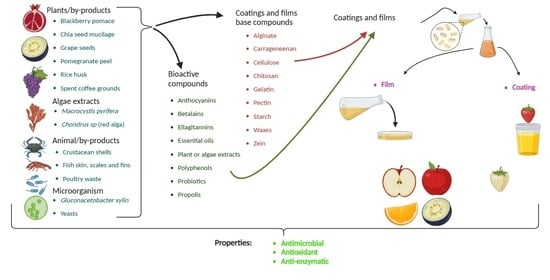
:1. Introduction
2. Biological Sources of the Compounds Used in Films and Coatings
3. Edible Film and Coating Functionalities
3.1. Bioactivity of the Edible Films and Coatings
3.1.1. Antimicrobial Properties
3.1.2. Antioxidant Properties
3.1.3. Anti-Enzymatic Capacity
3.2. Physical Properties of Edible Films and Coatings
3.3. Other Properties
3.4. Pitfalls of the Bioactives Present in Edible Films and Coatings
4. Health Effects of Edible Films and Coatings
5. Biodegradability of the Films
6. Innovations in the Edible Film and Coating Industry
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent Advances in Edible Coating of Food Products and Its Legislations: A Review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Thakur, N.; Kajla, P.; Kumar, M.; Trif, M. Natural Antimicrobials as Additives for Edible Food Packaging Applications: A Review. Foods 2021, 10, 2282. [Google Scholar] [CrossRef] [PubMed]
- Andriani, V.; Abyor Handayani, N. Recent Technology of Edible Coating Production: A Review. Mater. Today Proc. 2023, 87, 200–206. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life: Review. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Braga, M.E.M. Edible Films and Coatings Based on Agrifood Residues: A New Trend in the Food Packaging Research. Curr. Opin. Food Sci. 2023, 50, 101006. [Google Scholar] [CrossRef]
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S. Advancements in Biodegradable Active Films for Food Packaging: Effects of Nano/Microcapsule Incorporation. Foods 2022, 11, 760. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Y.; Bai, R.; Zhang, X.; Yuan, L.; Liu, J. Preparation of PH-Sensitive and Antioxidant Packaging Films Based on κ-Carrageenan and Mulberry Polyphenolic Extract. Int. J. Biol. Macromol. 2019, 134, 993–1001. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Chen, H. Characterization and Functional Properties of a Pectin/Tara Gum Based Edible Film with Ellagitannins from the Unripe Fruits of Rubus Chingii Hu. Food Chem. 2020, 325, 126964. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysac-charide-Based Materials as Advanced Food Packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.; Singh, K.; Tiwary, A.K.; Rana, V. Optimization of Microwave Assisted Maillard Reaction to Fabricate and Evaluate Corn Fiber Gum-Chitosan IPN Films. Food Hydrocoll. 2015, 44, 260–276. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Rahmati, E. Effect of Edible Coatings on the Shelf-Life of Fresh Strawberries: A Comparative Study Using TOPSIS-Shannon Entropy Method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- Aydogdu, A.; Radke, C.J.; Bezci, S.; Kirtil, E. Characterization of Curcumin Incorporated Guar Gum/Orange Oil Antimicrobial Emulsion Films. Int. J. Biol. Macromol. 2020, 148, 110–120. [Google Scholar] [CrossRef]
- Amalraj, A.; Raj, K.K.J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, Characterization, and Antimicrobial Activity of Chitosan/Gum Arabic/Polyethylene Glycol Composite Films Incorporated with Black Pepper Essential Oil and Ginger Es-sential Oil as Potential Packaging and Wound Dressing Materials. Adv. Compos. Hybrid. Mater. 2020, 3, 485–497. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Xia, K.; Liu, X.; Zhang, X. Preparation and Application of Edible Agar-Based Composite Films Modified by Cellulose Nanocrystals. Food Packag. Shelf Life 2022, 34, 100936. [Google Scholar] [CrossRef]
- Duong, N.T.C.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. An Inovative Single Step of Cross-Linked Alginate-Base Edible Coating for Mantaining Postharvest Quality and reducing Chilling Injury in Rose Apple Cv “Tabtim-chan” (Syzygium Samarangenese). Sci. Hortic. 2022, 292, 110648. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Salama, H.E. Development of Alginate-Based Edible Coatings of Optimized UV-Barrier Properties by Response Surface Methodology for Food Packaging Applications. Int. J. Biol. Macromol. 2022, 212, 294–302. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S. Development of Active Edible Coating of Alginate and Aloe Vera Enriched with Frankincense Oil for Retarding the Senescence of Green Capsicums. LWT 2021, 145, 111341. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Salama, H.E. Developing Multifunctional Edible Coatings Based on Alginate for Active Food Packaging. Int. J. Biol. Macromol. 2021, 190, 837–844. [Google Scholar] [CrossRef]
- Joshi, P.; Becerra-Mora, N.; Vargas-Lizarazo, A.Y.; Kohli, P.; Fisher, D.J.; Choudhary, R. Use of Edible Alginate and Limonene-Liposome Coatings for Shelf-Life Improvement of Blackberries. Future Foods 2021, 4, 100091. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Chen, C.; Gan, Z.; Chen, J.; Wan, C. (Craig) Loquat Leaf Extract and Alginate Based Green Composite Edible Coating for Preserving the Postharvest Quality of Nanfeng Tangerines. Sustain. Chem. Pharm. 2022, 27, 100674. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kulandhaivelu, S.V.; Roy, S. Alginate/Carboxymethyl Cellulose/Starch-Based Active Coating with Grapefruit Seed Extract to Extend the Shelf Life of Green Chilli. Ind. Crops Prod. 2023, 199, 116752. [Google Scholar] [CrossRef]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of Hydroxyethyl Cellulose and Sodium Alginate Edible Coating Containing Asparagus Waste Extract on Postharvest Quality of Strawberry Fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- Hira, N.; Mitalo, O.W.; Okada, R.; Sangawa, M.; Masuda, K.; Fujita, N.; Ushijima, K.; Akagi, T.; Kubo, Y. The Effect of Lay-er-by-Layer Edible Coating on the Shelf Life and Transcriptome of ‘Kosui’ Japanese Pear Fruit. Postharvest Biol. Technol. 2022, 185, 111787. [Google Scholar] [CrossRef]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of Edible Coating Enriched with Natural Antioxidant Extract and Bergamot Essential Oil on the Shelf Life of Strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef]
- Hashemi, M.; Dastjerdi, A.M.; Mirdehghan, S.H.; Shakerardekani, A.; Golding, J.B. Incorporation of Zataria multiflora Boiss Essential Oil into Gum Arabic Edible Coating to Maintain the Quality Properties of Fresh In-Hull Pistachio (Pistacia vera L.). Food Packag. Shelf Life 2021, 30, 100724. [Google Scholar] [CrossRef]
- Shakir, M.S.; Ejaz, S.; Hussain, S.; Ali, S.; Sardar, H.; Azam, M.; Ullah, S.; Khaliq, G.; Saleem, M.S.; Nawaz, A.; et al. Synergistic Effect of Gum Arabic and Carboxymethyl Cellulose as Biocomposite Coating Delays Senescence in Stored Tomatoes by Regulating Antioxidants and Cell Wall Degradation. Int. J. Biol. Macromol. 2022, 201, 641–652. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Boonyaritthongchai, P.; Buanong, M.; Supapvanich, S.; Wongs-Aree, C. Chitosan- and κ-Carrageenan-Based Composite Coating on Dragon Fruit (Hylocereus undatus) Pretreated with Plant Growth Regulators Maintains Bract Chlorophyll and Fruit Edibility. Sci. Hortic. 2021, 281, 109916. [Google Scholar] [CrossRef]
- Wani, S.M.; Gull, A.; Ahad, T.; Malik, A.R.; Ganaie, T.A.; Masoodi, F.A.; Gani, A. Effect of Gum Arabic, Xanthan and Carrageenan Coatings Containing Antimicrobial Agent on Postharvest Quality of Strawberry: Assessing the Physicochemical, Enzyme Activity and Bioactive Properties. Int. J. Biol. Macromol. 2021, 183, 2100–2108. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Q.; Liang, X.; Fang, S. Fabrication of Colloidal Stable Gliadin-Casein Nanoparticles for the Encapsulation of Natamycin: Molecular Interactions and Antifungal Application on Cherry Tomato. Food Chem. 2022, 391, 133288. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hu, Q.; Liang, X.; Chen, J.; Huan, C.; Fang, S. Methyl Jasmonate Encapsulated in Protein-Based Nanoparticles to Enhance Water Dispersibility and Used as Coatings to Improve Cherry Tomato Storage. Food Packag. Shelf Life 2022, 33, 100925. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Rahmati-Joneidabad, M.; Noshad, M. Effect of Chia Seed Mucilage/Bacterial Cellulose Edible Coating on Bioactive Compounds and Antioxidant Activity of Strawberries during Cold Storage. Int. J. Biol. Macromol. 2021, 190, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vishakha, K.; Das, S.; Chakraborty, D.; Ganguli, A. Carboxymethyl Cellulose and Cardamom Oil in a Nanoemulsion Edible Coating Inhibit the Growth of Foodborne Pathogens and Extend the Shelf Life of Tomatoes. Biocatal. Agric. Biotechnol. 2022, 42, 102369. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.Y.; Kim, J.; Moon, K.D. Effect of Edible Coating with Morus alba Root Extract and Carboxymethyl Cellulose for Enhancing the Quality and Preventing the Browning of Banana (Musa acuminata Cavendish) during Storage. Food Packag. Shelf Life 2022, 31, 100809. [Google Scholar] [CrossRef]
- Mahardiani, L.; Larasati, R.; Susilowati, E.; Hastuti, B.; Azizah, N.L. Potential Edible Coating of Pectin Obtained from Banana Peel for Fruit Preservation. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing Ltd.: Boston, MA, USA, 2021; Volume 1912, p. 012019. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of Carboxymethyl Cellulose- and Pectin-Based Active Edible Coatings in Preservation of Fruits and Vegetables: A Review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Picha, D.H.; Ferguson, M.H.; Ndukwe, I.E.; Azadi, P. Comparative Performance of Bio-Based Coatings For-mulated with Cellulose, Chitin, and Chitosan Nanomaterials Suitable for Fruit Preservation. Carbohydr. Polym. 2021, 259, 117764. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin Doped Functionalized Cellulose Nanofibers Based Edible Chitosan Coating on Kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef]
- Miranda, M.; Sun, X.; Marín, A.; dos Santos, L.C.; Plotto, A.; Bai, J.; Benedito Garrido Assis, O.; David Ferreira, M.; Baldwin, E. Nano- and Micro-Sized Carnauba Wax Emulsions-Based Coatings Incorporated with Ginger Essential Oil and Hydroxypropyl Methylcellulose on Papaya: Preservation of Quality and Delay of Post-Harvest Fruit Decay. Food Chem. X 2022, 13, 100249. [Google Scholar] [CrossRef]
- Sousa, F.F.; Pinsetta Junior, J.S.; Oliveira, K.T.E.F.; Rodrigues, E.C.N.; Andrade, J.P.; Mattiuz, B.H. Conservation of ‘Palmer’ Mango with an Edible Coating of Hydroxypropyl Methylcellulose and Beeswax. Food Chem. 2021, 346, 128925. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Y. Fabrication of Thermally and Mechanically Stable Superhydrophobic Coatings for Cellulose-Based Sub-strates with Natural and Edible Ingredients for Food Applications. Food Hydrocoll. 2021, 120, 106877. [Google Scholar] [CrossRef]
- Isopencu, G.O.; Stoica-Guzun, A.; Busuioc, C.; Stroescu, M.; Deleanu, I.M. Development of Antioxidant and Antimicrobial Edible Coatings Incorporating Bacterial Cellulose, Pectin, and Blackberry Pomace. Carbohydr. Polym. Technol. Appl. 2021, 2, 100057. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Farag, M.A.; Shao, P. Functionalized Cellulose Nanocrystal Embedded into Citrus Pectin Coating Improves Its Barrier, Antioxidant Properties and Potential Application in Food. Food Chem. 2023, 401, 134079. [Google Scholar] [CrossRef] [PubMed]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Application of Pregelatinized Corn Starch and Basil Essential Oil Edible Coating with Cellulose Nanofiber as Pickering Emulsion Agent to Prevent Quality-Quantity Loss of Mandarin Orange. Food Packag. Shelf Life 2023, 35, 101010. [Google Scholar] [CrossRef]
- Nazoori, F.; Mollai, S.; Sobhani, F.; Mirdehghan, S.H.; Sahhafi, S.R. Carboxymethyl Cellulose and Carnauba Wax Treatments Kept the Pomegranate Fruit (Punica granatum L.) Quality during Cold Storage via Improving Enzymatic Defense System and Bioactive Compounds. Sci. Hortic. 2023, 309, 111645. [Google Scholar] [CrossRef]
- Yang, C.; Lee, F.W.; Cheng, Y.J.; Chu, Y.Y.; Chen, C.N.; Kuan, Y.C. Chitosan Coating Formulated with Citric Acid and Pomelo Extract Retards Pericarp Browning and Fungal Decay to Extend Shelf Life of Cold-Stored Lychee. Sci. Hortic. 2023, 310, 111735. [Google Scholar] [CrossRef]
- Espinal-Hernández, P.; Colinas-León, M.T.; Ybarra-Moncada, M.C.; Méndez-Zúñiga, S.M.; Corrales-García, J. Postharvest Effects of 1-Mcp and Chitosan/Oleic Acid Coating in Pitaya (Stenocereus griseus h.). J. Prof. Assoc. Cactus Dev. 2021, 23, 43–57. [Google Scholar] [CrossRef]
- Das, S.K.; Vishakha, K.; Das, S.; Ganguli, A. Antibacterial and Antibiofilm Activities of Nanoemulsion Coating Prepared by Using Caraway Oil and Chitosan Prolongs the Shelf Life and Quality of Bananas. Appl. Food Res. 2023, 3, 100300. [Google Scholar] [CrossRef]
- Shaukat, M.N.; Palmeri, R.; Restuccia, C.; Parafati, L.; Fallico, B. Glycerol Ginger Extract Addition to Edible Coating Formu-lation for Preventing Oxidation and Fungal Spoilage of Stored Walnuts. Food Biosci. 2023, 52, 102420. [Google Scholar] [CrossRef]
- Popescu, P.A.; Palade, L.M.; Nicolae, I.C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef]
- Aparicio-García, P.F.; Ventura-Aguilar, R.I.; Del Río-García, J.C.; Hernández-López, M.; Guillén-Sánchez, D.; Salazar-Piña, D.A.; Ramos-García, M.d.L.; Bautista-Baños, S. Edible Chitosan/Propolis Coatings and Their Effect on Ripening, Development of Aspergillus Flavus, and Sensory Quality in Fig Fruit, during Controlled Storage. Plants 2021, 10, 112. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, Q.; Yang, Q.; Zhou, X.; Cheng, S.; Wei, B.; Li, J.; Ji, S. Influence of Combined Edible Coating with Chitosan and Tea Polyphenol on the Quality Deterioration and Health-Promoting Compounds in Harvested Broccoli. Food Bioproc. Technol. 2022, 15, 407–420. [Google Scholar] [CrossRef]
- Tran, V.T.; Kingwascharapong, P.; Tanaka, F.; Tanaka, F. Effect of Edible Coatings Developed from Chitosan Incorporated with Tea Seed Oil on Japanese Pear. Sci. Hortic. 2021, 288, 110314. [Google Scholar] [CrossRef]
- ValizadehKaji, B.; Seyfori, P.; Abbasifar, A. Effect of Chitosan and Thymol on Physicochemical and Qualitative Properties of Table Grape Fruits during the Postharvest Period. Biologia 2023, 78, 279–289. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S.; Qazi, I.M.; Durrani, Y.; Sarkhosh, A.; Hussain, I.; Brecht, J.K. Pre-Storage Chitosan-Thyme Oil Coating Control Anthracnose in Mango Fruit. Sci. Hortic. 2021, 284, 110139. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Dong, Y.; Wang, Y. Effect of Chitosan Coating Incorporated with Torreya grandis Essential Oil on the Quality and Physiological Attributes of Loquat Fruit. J. Food Meas. Charact. 2022, 16, 2820–2830. [Google Scholar] [CrossRef]
- Erceg, T.; Šovljanski, O.; Stupar, A.; Ugarković, J.; Aćimović, M.; Pezo, L.; Tomić, A.; Todosijević, M. A Comprehensive Ap-proach to Chitosan-Gelatine Edible Coating with β-Cyclodextrin/Lemongrass Essential Oil Inclusion Complex—Characterization and Food Application. Int. J. Biol. Macromol. 2023, 228, 400–410. [Google Scholar] [CrossRef]
- Moreira Pereira, E.; Dellinghausen Borges, C.; dos Santos Formiga, A.; Sidnaldo Pinsetta Junior, J.; Mattiuz, B.H.; Santos Monteiro, S. Conservation of Red Guava “Pedro Sato” Using Chitosan and Gelatin-Based Coatings Produced by the Lay-er-by-Layer Technique. Process Biochem. 2022, 121, 35–44. [Google Scholar] [CrossRef]
- Sekarina, A.S.; Supriyadi; Munawaroh, H.S.H.; Susanto, E.; Show, P.L.; Ningrum, A. Effects of Edible Coatings of Chitosan-Fish Skin Gelatine Containing Black Tea Extract on Quality of Minimally Processed Papaya during Refrigerated Storage. Car-Bohydr. Polym. Technol. Appl. 2023, 5, 100287. [Google Scholar] [CrossRef]
- Bhan, C.; Asrey, R.; Meena, N.K.; Rudra, S.G.; Chawla, G.; Kumar, R.; Kumar, R. Guar Gum and Chitosan-Based Composite Edible Coating Extends the Shelf Life and Preserves the Bioactive Compounds in Stored Kinnow Fruits. Int. J. Biol. Macromol. 2022, 222, 2922–2935. [Google Scholar] [CrossRef]
- Le, K.H.; La, D.D.; Nguyen, P.T.M.; Nguyen, M.D.B.; Vo, A.T.K.; Nguyen, M.T.H.; Tran, D.L.; Chang, S.W.; Nguyen, X.H.; Duc Nguyen, D. Fabrication of Cleistocalyx Operculatus Extracts/Chitosan/Gum Arabic Composite as an Edible Coating for Preservation of Banana. Prog. Org. Coat. 2021, 161, 106550. [Google Scholar] [CrossRef]
- Choo, K.S.O.; Bollen, M.; Ravensdale, J.T.; Dykes, G.A.; Coorey, R. Effect of Chitosan and Gum Arabic with Natamycin on the Aroma Profile and Bacterial Community of Australian Grown Black Périgord Truffles (Tuber melansoporum) during Storage. Food Microbiol. 2021, 97, 103743. [Google Scholar] [CrossRef]
- Chen, K.; Tian, R.; Xu, G.; Wu, K.; Liu, Y.; Jiang, F. Characterizations of Konjac Glucomannan/Curdlan Edible Coatings and the Preservation Effect on Cherry Tomatoes. Int. J. Biol. Macromol. 2023, 232, 123359. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, S. Preservation of Mango Fruit Quality Using Fucoidan Coatings. LWT 2021, 143, 111150. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, S.; Zou, Y.; Lei, L.; Zhou, Y.; Wang, D.; Ye, F.; Zhao, G. Coating Peanut Shell Lignin Nanospheres with Gelatin via Non-Covalent Adsorption: Key Parameters, Consequences, and Underlying Interactions. Int. J. Biol. Macromol. 2023, 233, 123607. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, E.; Rousta, E. Shelf-Life Extension of Tomato (Solanum lycopersicum L.) Using an Edible Coating of Bitter Almond Gum-Fish Gelatin Conjugates. Prog. Org. Coat. 2022, 170, 106980. [Google Scholar] [CrossRef]
- Rather, J.A.; Makroo, H.A.; Showkat, Q.A.; Majid, D.; Dar, B.N. Recovery of Gelatin from Poultry Waste: Characteristics of the Gelatin and Lotus Starch-Based Coating Material and Its Application in Shelf-Life Enhancement of Fresh Cherry Tomato. Food Packag. Shelf Life 2022, 31, 100775. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, T.L.; Nguyen, P.L.; Baranyai, L.; Trinh, K.S. Effect of Electrolyzed Cassava Starch-Gelatin Coating on Bio-chemical Properties and Ripening of Banana (Musa acuminata L.) Fruits. Pol. J. Food Nutr. Sci. 2022, 72, 263–272. [Google Scholar] [CrossRef]
- Qambrani, S.; Talpur, F.N.; Panhwar, A.A.; Afridi, H.I.; Talpur, M.K.; Khan, A.; Hab, S.A. Development of Guar Gum-Based Coating with Castor Oil for Improved Postharvest Quality of Fresh Mangoes Using Response Surface Methodology. Appl. Food Res. 2022, 2, 100220. [Google Scholar] [CrossRef]
- Ghosh, T.; Katiyar, V. Nanochitosan Functionalized Hydrophobic Starch/Guar Gum Biocomposite for Edible Coating Ap-plication with Improved Optical, Thermal, Mechanical, and Surface Property. Int. J. Biol. Macromol. 2022, 211, 116–127. [Google Scholar] [CrossRef]
- Mohd Suhaimi, N.I.; Mat Ropi, A.A.; Shaharuddin, S. Safety and Quality Preservation of Starfruit (Averrhoa carambola) at Ambient Shelf Life Using Synergistic Pectin-Maltodextrin-Sodium Chloride Edible Coating. Heliyon 2021, 7, e06279. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Cao, J.; Wang, Y.; Huang, L.; Chen, J.; Wu, J.; Zhang, H.; Chen, Y.; Sun, C. Preparation and Characterization of Pectin-Based Edible Coating Agent Encapsulating Carvacrol/HPβCD Inclusion Complex for Inhibiting Fungi. Food Hydrocoll. 2022, 125, 107374. [Google Scholar] [CrossRef]
- Hernández-Carrillo, J.G.; Orta-Zavalza, E.; González-Rodríguez, S.E.; Montoya-Torres, C.; Sepúlveda-Ahumada, D.R.; Ortiz-Rivera, Y. Evaluation of the Effectivity of Reuterin in Pectin Edible Coatings to Extend the Shelf-Life of Strawberries during Cold Storage. Food Packag. Shelf Life 2021, 30, 100760. [Google Scholar] [CrossRef]
- Aguilar-Veloz, L.M.; Calderón-Santoyo, M.; Carvajal-Millan, E.; Martínez-Robinson, K.; Ragazzo-Sánchez, J.A. Artocarpus heterophyllus Lam. Leaf Extracts Added to Pectin--Based Edible Coating for Alternaria sp. Control in Tomato. LWT 2022, 156, 113022. [Google Scholar] [CrossRef]
- Li, K.; Tang, B.; Zhang, W.; Tu, X.; Ma, J.; Xing, S.; Shao, Y.; Zhu, J.; Lei, F.; Zhang, H. A Novel Approach for Authentication of Shellac Resin in the Shellac-Based Edible Coatings: Contain Shellac or Not in the Fruit Wax Preservative Coating. Food Chem. X 2022, 14, 100349. [Google Scholar] [CrossRef] [PubMed]
- Pongsri, R.; Aiamla-or, S.; Srilaong, V.; Uthairatanakij, A.; Jitareerat, P. Impact of Electron-Beam Irradiation Combined with Shellac Coating on the Suppression of Chlorophyll Degradation and Water Loss of Lime Fruit during Storage. Postharvest Biol. Technol. 2021, 172, 111364. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Ye, J. Decolorizing Shellac Incorporated with Natural Antibacterial Juglone from Walnut Green Husk Extract for Preserving the Postharvest Quality of Wichita Pecans (Carya illinoinensis [Wangenh.] K. Koch) during Storage. Sci. Hortic. 2022, 304, 111313. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Z.; Li, K.; Li, K.; Liu, L.; Zhang, W.; Xu, J.; Tu, X.; Du, L.; Zhang, H. Novel Edible Coating Based on Shellac and Tannic Acid for Prolonging Postharvest Shelf Life and Improving Overall Quality of Mango. Food Chem. 2021, 354, 129510. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Li, H.; Alomgir Hossen, M.; Sameen, D.E.; Dai, J.; Qin, W.; Lee, K.J. Synthesis and Properties of Core-Shell Thymol-Loaded Zein/Shellac Nanoparticles by Coaxial Electrospray as Edible Coatings. Mater. Des. 2021, 212, 110214. [Google Scholar] [CrossRef]
- Gupta, V.; Thakur, R.; Barik, M.; Das, A.B. Effect of High Amylose Starch-Natural Deep Eutectic Solvent Based Edible Coating on Quality Parameters of Strawberry during Storage. J. Agric. Food Res. 2023, 11, 100487. [Google Scholar] [CrossRef]
- Chettri, S.; Sharma, N.; Mohite, A.M. Utilization of Lima Bean Starch as an Edible Coating Base Material for Sapota Fruit Shelf-Life Enhancement. J. Agric. Food Res. 2023, 12, 100615. [Google Scholar] [CrossRef]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Strawberry Preservation Using Combination of Yam Bean Starch, Agar-wood Aetoxylon bouya Essential Oil, and Calcium Propionate Edible Coating during Cold Storage Evaluated by TOP-SIS-Shannon Entropy. Prog. Org. Coat. 2023, 175, 107347. [Google Scholar] [CrossRef]
- Trinh, B.M.; Smith, M.; Mekonnen, T.H. A Nanomaterial-Stabilized Starch-Beeswax Pickering Emulsion Coating to Extend Produce Shelf-Life. J. Chem. Eng. 2022, 431, 133905. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Arfin, M.S.; Rahman, M.A.; Molla, M.M.; Sabuz, A.A. Influence of Novel Coconut Oil and Beeswax Edible Coating and MAP on Postharvest Shelf Life and Quality Attributes of Lemon at Low Temperature. Meas. Food 2023, 10, 100084. [Google Scholar] [CrossRef]
- Sinha, A.; Gill, P.P.S.; Jawandha, S.K.; Kaur, P.; Grewal, S.K. Salicylic Acid Enriched Beeswax Coatings Suppress Fruit Softening in Pears by Modulation of Cell Wall Degrading Enzymes under Different Storage Conditions. Food Packag. Shelf Life 2022, 32, 100821. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Gu, G.; Luo, Y.; Zografos, A.; Minas, I.; Nou, X. Edible and Water-Soluble Corn Zein Coating Impregnated with Nisin for Listeria monocytogenes Reduction on Nectarines and Apples. Postharvest Biol. Technol. 2022, 185, 111811. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.; Duarte, L.G.R.; Silva, Y.B.B.; Milan, E.P.; Santos, H.V.; Moura, T.C.; Bandini, V.P.; Vitolano, L.E.S.; Nobre, J.J.C.; Moreira, C.T.; et al. Novel Approach for Improving Papaya Fruit Storage with Carnauba Wax Nanoemulsion in Combination with Syzigium aromaticum and Mentha spicata Essential Oils. Coatings 2023, 13, 847. [Google Scholar]
- Celik, N.; Kiremitler, N.B.; Ruzi, M.; Onses, M.S. Waxing the Soot: Practical Fabrication of All-Organic Superhydrophobic Coatings from Candle Soot and Carnauba Wax. Prog. Org. Coat. 2021, 153, 106169. [Google Scholar] [CrossRef]
- Miranda, M.; Ribeiro, M.D.M.M.; Spricigo, P.C.; Pilon, L.; Mitsuyuki, M.C.; Correa, D.S.; Ferreira, M.D. Carnauba Wax Nanoemulsion Applied as an Edible Coating on Fresh Tomato for Postharvest Quality Evaluation. Heliyon 2022, 8, e09803. [Google Scholar] [CrossRef]
- Wang, X.; Chen, K.; Liu, Y.; He, R.; Wang, Q. Preparation and Application of Biodegradable and Superhydrophobic Polylactic Acid/Carnauba Wax Coating. Prog. Org. Coat. 2023, 177, 107434. [Google Scholar] [CrossRef]
- Phothisuwan, S.; Koomhin, P.; Matan, N.; Matan, N. Quality Maintenance of Salacca Fruit with a Carnauba Wax Coating Containing Orange Oil and Detection of Sensory Perception Improvement with Electroencephalography to Appraise Brain Responses. LWT 2021, 147, 111628. [Google Scholar] [CrossRef]
- Torun, M.; Ozdemir, F. Milk Protein and Zein Coatings over Peeled Garlic Cloves to Extend Their Shelf Life. Sci. Hortic. 2022, 291, 110571. [Google Scholar] [CrossRef]
- Elsayed, N.; Hassan, A.A.-M.; Abdelaziz, S.M.; Abdeldaym, E.A.; Darwish, O.S. Effect of Whey Protein Edible Coating Incorporated with Mango Peel Extract on Postharvest Quality, Bioactive Compounds and Shelf Life of Broccoli. Horticulturae 2022, 8, 770. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, C.S. Edible Composite Bi-Layer Coating Based on Whey Protein Isolate, Xanthan Gum and Clove Oil for Prolonging Shelf Life of Tomatoes. Meas. Food 2021, 2, 100005. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, L.; Tang, B.; Qin, J.; Wu, K.; Jiang, F. Physical, Structural, and Water Barrier Properties of Emulsified Blend Film Based on Konjac Glucomannan/Agar/Gum Arabic Incorporating Virgin Coconut Oil. LWT 2022, 154, 112683. [Google Scholar] [CrossRef]
- Zhang, R.; Zhai, X.; Wang, W.; Hou, H. Preparation and Evaluation of Agar/Maltodextrin-Beeswax Emulsion Films with Various Hydrophilic-Lipophilic Balance Emulsifiers. Food Chem. 2022, 384, 132541. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, W.; Zhang, J.; Xie, G.; Xiong, T.; Xu, H. Preparation and Characterization of Sodium Alginate/Gelatin/Ag Nano-composite Antibacterial Film and Its Application in the Preservation of Tangerine. Food Packag. Shelf Life 2022, 33, 100928. [Google Scholar] [CrossRef]
- Medeiros, J.A.; Otoni, C.G.; Niro, C.M.; Sivieri, K.; Barud, H.S.; Guimarães, F.E.G.; Alonso, J.D.; Azeredo, H.M.C. Alginate Films as Carriers of Probiotic Bacteria and Pickering Emulsion. Food Packag. Shelf Life 2022, 34, 100987. [Google Scholar] [CrossRef]
- Dursun Capar, T. Characterization of Sodium Alginate-Based Biodegradable Edible Film Incorporated with Vitis Vinifera Leaf Extract: Nano-Scaled by Ultrasound-Assisted Technology. Food Packag. Shelf Life 2023, 37, 101068. [Google Scholar] [CrossRef]
- Zhang, Y.; Man, J.; Li, J.; Xing, Z.; Zhao, B.; Ji, M.; Xia, H.; Li, J. Preparation of the Alginate/Carrageenan/Shellac Films Rein-forced with Cellulose Nanocrystals Obtained from Enteromorpha for Food Packaging. Int. J. Biol. Macromol. 2022, 218, 519–532. [Google Scholar] [CrossRef]
- Shi, S.; Xu, X.; Ren, Y.; Zhang, H.; Du, X.; Li, H.; Xia, X. Beeswax Coating Improves the Hydrophobicity of Sodium Alginate/Anthocyanin/Cellulose Nanocrystal Indicator Film. Food Hydrocoll. 2023, 144, 108930. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, H.; Fu, Y.; Chang, C.; Wu, J. Sodium Alginate/Gum Arabic/Glycerol Multicomponent Edible Films Loaded with Natamycin: Study on Physicochemical, Antibacterial, and Sweet Potatoes Preservation Properties. Int. J. Biol. Macromol. 2022, 213, 1068–1077. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Inhibitory Effect of Reuterin-Producing Limosilactobacillus reuteri and Edible Alginate-Konjac Gum Film against Foodborne Pathogens and Spoilage Microorganisms. Food Biosci. 2023, 52, 102443. [Google Scholar] [CrossRef]
- Hadi, A.; Nawab, A.; Alam, F.; Zehra, K. Alginate/Aloe Vera Films Reinforced with Tragacanth Gum. Food Chem. 2022, 4, 100105. [Google Scholar] [CrossRef]
- Bishnoi, S.; Trifol, J.; Moriana, R.; Mendes, A.C. Adjustable Polysaccharides-Proteins Films Made of Aqueous Wheat Proteins and Alginate Solutions. Food Chem. 2022, 391, 133196. [Google Scholar] [CrossRef] [PubMed]
- Abdillah, A.A.; Charles, A.L. Characterization of a Natural Biodegradable Edible Film Obtained from Arrowroot Starch and Iota-Carrageenan and Application in Food Packaging. Int. J. Biol. Macromol. 2021, 191, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Sogut, E.; Filiz, B.E.; Seydim, A.C. Whey Protein Isolate- and Carrageenan-Based Edible Films as Carriers of Different Probiotic Bacteria. J. Dairy Sci. 2022, 105, 4829–4842. [Google Scholar] [CrossRef]
- Mortazavi Moghadam, F.A.; Khoshkalampour, A.; Mortazavi Moghadam, F.A.; PourvatanDoust, S.; Naeijian, F.; Ghorbani, M. Preparation and Physicochemical Evaluation of Casein/Basil Seed Gum Film Integrated with Guar Gum/Gelatin Based Nanogel Containing Lemon Peel Essential Oil for Active Food Packaging Application. Int. J. Biol. Macromol. 2023, 224, 786–796. [Google Scholar] [CrossRef]
- Sood, A.; Saini, C.S. Utilization of Peel of White Pomelo for the Development of Pectin Based Biodegradable Composite Films Blended with Casein and Egg Albumen. Food Chem. Adv. 2022, 1, 100054. [Google Scholar] [CrossRef]
- Zioga, M.; Papantonopoulou, G.; Evageliou, V. High Internal Phase Emulsions and Edible Films with High Methoxyl Pectin and Pea Protein Isolate or Sodium Caseinate. Food Hydrocoll. 2023, 140, 108605. [Google Scholar] [CrossRef]
- More, P.R.; Pegu, K.; Arya, S.S. Development and Characterization of Taro Starch-Casein Composite Bioactive Films Func-tionalized by Micellar Pomegranate Peel Extract (MPPE). Int. J. Biol. Macromol. 2022, 220, 1060–1071. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.K.; Yue, L.N.; Xu, L.; Qian, J.Y.; He, X.D. Variation of Blending Ratio and Drying Temperature Optimize the Physical Properties and Compatibility of HPMC/Curdlan Films. Carbohydr. Polym. 2022, 296, 119951. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Man, T.; Xiong, X.; Wang, Y.; Duan, X.; Xiong, X. HPMC Films Functionalized by Zein/Carboxymethyl Tamarind Gum Stabilized Pickering Emulsions: Influence of Carboxymethylation Degree. Int. J. Biol. Macromol. 2023, 238, 124053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Y. Physical and Antimicrobial Properties of Zein and Methyl Cellulose Composite Films with Plasticizers of Oleic Acid and Polyethylene Glycol. LWT 2021, 140, 110811. [Google Scholar] [CrossRef]
- Chetia, P.; Bharadwaj, C.; Purbey, R.; Bora, D.; Yadav, A.; Lal, M.; Rajulu, A.V.; Sadiku, E.R.; Selvam, S.P.; Jarugala, J. Influence of Silylated Nano Cellulose Reinforcement on the Mechanical, Water Resistance, Thermal, Morphological and Antibacterial Properties of Soy Protein Isolate (SPI)-Based Composite Films. Int. J. Biol. Macromol. 2023, 242, 124861. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Wane, B.M.; Nordin, N.; Noor Hasnan, N.Z.; Talib, R.A.; Karyadi, J.N.W. Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers 2021, 13, 4060. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Antoniewska, A.; Rutkowska, J.; Vázquez, M. Evaluation of Easy-Removing Antioxidant Films of Chitosan with Melaleuca Alternifolia Essential Oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [CrossRef]
- Mu, R.; Bu, N.; Yuan, Y.; Pang, J.; Ma, C.; Wang, L. Development of Chitosan/Konjac Glucomannan/Tragacanth Gum Tri-Layer Food Packaging Films Incorporated with Tannic Acid and ε-Polylysine Based on Mussel-Inspired Strategy. Int. J. Biol. Mac-romol. 2023, 242, 125100. [Google Scholar] [CrossRef]
- Adair, P.; Sriprom, P.; Narkrugsa, W.; Phumjan, L.; Manamoongmongkol, K.; Permana, L.; Assawasaengrat, P. Preparation, Characterization, and Antimicrobial Activity of Xyloglucan-Chitosan Film from Tamarind (Tamarind indica L.) Seed Kernel. Prog. Org. Coat. 2023, 179, 107486. [Google Scholar] [CrossRef]
- Don, T.M.; Liu, L.M.; Chen, M.; Huang, Y.C. Crosslinked Complex Films Based on Chitosan and Ulvan with Antioxidant and Whitening Activities. Algal Res. 2021, 58, 102423. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Mirzapour, A.; Ghiasi, F.; Eskandari, M.H.; Moosavi-Nasab, M.; Hosseini, S.M.H. Development and Characterization of Gelatin and Persian Gum Composite Edible FIlms through Complex Coacervation. LWT 2022, 153, 112422. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Yu, R.; Zheng, H.; Wang, P. Acylated Pectin/Gelatin-Based Films Incorporated with Alkylated Starch Crystals: Characterization, Antioxidant and Antibacterial Activities, and Coating Preservation Effects on Golden Pomfret. Int. J. Biol. Macromol. 2023, 241, 124532. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Du, Y.; Yuan, S.; Pang, J. Dihydromyricetin Incorporated Active Films Based on Konjac Glucomannan and Gellan Gum. Int. J. Biol. Macromol. 2021, 180, 385–391. [Google Scholar] [CrossRef]
- Agarwal, N.; Jyoti; Thakur, M.; Mishra, B.B.; Singh, S.P. Preparation and Characterization of Biodegradable Films Based on Levan Polysaccharide Blended with Gellan Gum. Environ. Technol. Innov. 2023, 31, 103231. [Google Scholar] [CrossRef]
- Hosseini, P.; Hojjatoleslamy, M.; Molavi, H. Investigation of the Mixing Ratio of Quince Seed Gum, Potato Starch and Gellan Gum on the Properties of the Resulting Film by Mixture Design. Int. J. Biol. Macromol. 2023, 237, 123869. [Google Scholar] [CrossRef]
- Najafian, N.; Aarabi, A.; Nezamzadeh-Ejhieh, A. Evaluation of Physicomechanical Properties of Gluten-Based Film Incorporated with Persian Gum and Guar Gum. Int. J. Biol. Macromol. 2022, 223, 1257–1267. [Google Scholar] [CrossRef]
- Tabatabaei, S.D.; Ghiasi, F.; Hashemi Gahruie, H.; Hosseini, S.M.H. Effect of Emulsified Oil Droplets and Glycerol Content on the Physicochemical Properties of Persian Gum-Based Edible Films. Polym. Test. 2022, 106, 107427. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Wahab, N.I.A. Corn Starch (Zea mays) Biopolymer Plastic Reaction in Combination with Sorbitol and Glycerol. Polymers 2021, 13, 242. [Google Scholar] [CrossRef]
- de Freitas, T.S.M.; Garcia, V.A.D.S.; Filgueiras, C.T.; Velasco, J.I.; Fakhouri, F.M. Production of Edible Films Based on Pea Starch with Incorporation of Active Compounds Obtained from the Purple Araçá (Psidium myrtoides). Polymers 2021, 13, 3134. [Google Scholar] [CrossRef]
- Erturk, N.; Biran AY, S. Effect of Ethylene Glycol and Glycerol Concentrations on Properties of Rye-Based Films. EJOSAT 2022, 34, 705–710. [Google Scholar] [CrossRef]
- Lintang, M.; Tandi, O.; Layuk, P.; Karouw, S.; Dirpan, A. Characterization Edible Films of Sago with Glycerol as a Plasticizer. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Boston, MA, USA, 2021; Volume 807, p. 022070. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Multilayer Antimicrobial Films Based on Starch and PLA with Superficially Incorporated Ferulic or Cinnamic Acids for Active Food Packaging Purposes. Food Chem. Adv. 2023, 2, 100250. [Google Scholar] [CrossRef]
- Pérez, P.F.; Resa, C.P.O.; Gerschenson, L.N.; Jagus, R.J. Addition of Zein for the Improvement of Physicochemical Properties of Antimicrobial Tapioca Starch Edible Film. Food Bioproc. Technol. 2021, 14, 262–271. [Google Scholar] [CrossRef]
- Alvarez-Perez, O.B.; Ventura-Sobrevilla, J.M.; Torres-León, C.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Aguilar-González, M.A.; Aguilar, C.N. Development and Characterization of Whey Protein Films Incorporated with Tarbush Polyphenols and Candelilla Wax. Food Biosci. 2022, 45, 101505. [Google Scholar] [CrossRef]
- Alipour, A.; Rahaiee, S.; Rajaei Litkohi, H.; Jamali, S.N.; Jafari, S.M. Development and Optimization of Whey Protein- Lepidium perfoliatum Gum Packaging Films: An Approach towards Antimicrobial and Biodegradable Films. Ind. Crops Prod. 2023, 196, 116447. [Google Scholar] [CrossRef]
- Jiang, L.; Ye, R.; Xie, C.; Wang, F.; Zhang, R.; Tang, H.; He, Z.; Han, J.; Liu, Y. Development of Zein Edible Films Containing Different Catechin/Cyclodextrin Metal-Organic Frameworks: Physicochemical Characterization, Antioxidant Stability and Release Behavior. LWT 2023, 173, 114306. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, J.; Duan, A.; Li, X. Pectin/Sodium Alginate/Xanthan Gum Edible Composite Films as the Fresh-Cut Package. Int. J. Biol. Macromol. 2021, 181, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Roshandel-hesari, N.; Mokaber-Esfahani, M.; Taleghani, A.; Akbari, R. Investigation of Physicochemical Properties, Anti-microbial and Antioxidant Activity of Edible Films Based on Chitosan/Casein Containing Origanum vulgare L. Essential Oil and Its Effect on Quality Maintenance of Cherry Tomato. Food Chem. 2022, 396, 133650. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Qin, S.; Han, S.; Qi, H. Antimicrobial, UV Blocking, Water-Resistant and Degradable Coatings and Packaging Films Based on Wheat Gluten and Lignocellulose for Food Preservation. Compos. B Eng. 2022, 238, 109868. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, G.; Yang, Y.; Zheng, B.; Zhang, S.; Li, X.; Tian, Y.; Peng, B. Development and Characterization of Levan/Pullulan/Chitosan Edible Films Enriched with ε-Polylysine for Active Food Packaging. Food Chem. 2022, 388, 132989. [Google Scholar] [CrossRef]
- Jin, T.Z.; Yadav, M.P.; Qi, P.X. Antimicrobial and Physiochemical Properties of Films and Coatings Prepared from Bio-Fiber Gum and Whey Protein Isolate Conjugates. Food Control 2023, 148, 109666. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Wang, T.; Li, Z.; Zou, X.; Huang, X.; Zhai, X.; Shi, J.; Shen, T.; Gong, Y.; et al. Novel Gellan Gum-Based Probiotic Film with Enhanced Biological Activity and Probiotic Viability: Application for Fresh-Cut Apples and Potatoes. Int. J. Biol. Macromol. 2023, 239, 124128. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Avila-Sosa, R. Starch Edible Films/Coatings Added with Carvacrol and Thymol: In Vitro and in Vivo Eval-uation against Colletotrichum gloeosporioides. Foods 2021, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Rohasmizah, H.; Azizah, M. Pectin-Based Edible Coatings and Nanoemulsion for the Preservation of Fruits and Vegetables: A Review. Appl. Food Res. 2022, 2, 100221. [Google Scholar] [CrossRef]
- Tiamiyu, Q.O.; Adebayo, S.E.; Yusuf, A.A. Gum Arabic Edible Coating and Its Application in Preservation of Fresh Fruits and Vegetables: A Review. Food Chem. Adv. 2023, 2, 100251. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Chen, L.; Liu, J.; Cao, J.; Jiang, W. Recent Advances in Guar Gum-Based Films or Coatings: Diverse Property Enhancement Strategies and Applications in Foods. Food Hydrocoll. 2023, 136, 108278. [Google Scholar]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the Functionality of Chitosan- and Alginate-Based Active Edible Coatings/Films for the Preservation of Fruits and Vegetables: A Review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.R.L.; Basumatary, I.B.; Nayak, A.; Dutta, D.; Konwar, J.; Purkayastha, M.D.; Mukherjee, A. Recent Progress in Pectin Extraction and Their Applications in Developing Films and Coatings for Sustainable Food Packaging: A Review. Int. J. Biol. Macromol. 2023, 239, 124281. [Google Scholar] [CrossRef]
- Anisha, G.S.; Augustianath, T.; Padmakumari, S.; Singhania, R.R.; Pandey, A.; Patel, A.K. Ulvan from Green Macroalgae: Bioactive Properties Advancing Tissue Engineering, Drug Delivery Systems, Food Industry, Agriculture and Water Treatment. Bioresour. Technol. Rep. 2023, 22, 101457. [Google Scholar] [CrossRef]
- Khedri, S.; Sadeghi, E.; Rouhi, M.; Delshadian, Z.; Mortazavian, A.M.; de Toledo Guimarães, J.; Fallah, M.; Mohammadi, R. Bioactive Edible Films: Development and Characterization of Gelatin Edible Films Incorporated with Casein Phosphopeptides. LWT 2021, 138, 110649. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Lee, N.A.; Bekhit, A.E.D.; Carne, A.; Vaskoska, R.; Vasiljevic, T. Active Edible Packaging Based on Milk Proteins: A Route to Carry and Deliver Nutraceuticals. Trends Food Sci. Technol. 2021, 111, 688–705. [Google Scholar]
- Aranda-Ledesma, N.E.; Bautista-Hernández, I.; Rojas, R.; Aguilar-Zárate, P.; Medina-Herrera, N.d.P.; Castro-López, C.; Guadalupe Martínez-Ávila, G.C. Candelilla Wax: Prospective Suitable Applications within the Food Field. LWT 2022, 159, 113170. [Google Scholar] [CrossRef]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine Fucoidans: Structural, Extraction, Biological Activities and Their Applications in the Food Industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar]
- Tan, M.T.H.; Eshaghi Gorji, M.; Toh, J.Y.L.; Park, A.Y.; Li, Y.; Gong, Z.; Li, D. Fucoidan from Fucus Versiculosus Can Inhibit Human Norovirus Replication by Enhancing the Host Innate Immune Response. J. Funct. Foods 2022, 95, 105149. [Google Scholar] [CrossRef]
- de Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba Wax Uses in Food–A Review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium Alginate-Based Edible Coating Containing Nanoemulsion of Citrus sinensis Essential Oil Eradicates Planktonic and Sessile Cells of Food-Borne Pathogens and Increased Quality Attributes of Tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Tumbarski, Y.; Petkova, N.; Todorova, M.; Ivanov, I.; Deseva, I.; Mihaylova, D.; Ibrahim, S.A. Effects of Pectin-based Edible Coatings Containing a Bacteriocin of Bacillus Methylotrophicus BM47 on the Quality and Storage Life of Fresh Blackberries. Ital. J. Food Sci. 2020, 32, 420–437. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, Y.; Zhang, W.; Shi, S.; Zhu, W.; Wang, R.; Zhang, L.; Chen, L.; Sun, J.; Pang, J.; et al. Advanced Konjac Glucoman-nan-Based Films in Food Packaging: Classification, Preparation, Formation Mechanism and Function. LWT 2021, 152, 112338. [Google Scholar] [CrossRef]
- Lara, G.; Yakoubi, S.; Villacorta, C.M.; Uemura, K.; Kobayashi, I.; Takahashi, C.; Nakajima, M.; Neves, M.A. Spray Technology Applications of Xanthan Gum-Based Edible Coatings for Fresh-Cut Lotus Root (Nelumbo nucifera). Food Res. Int. 2020, 137, 109723. [Google Scholar] [CrossRef]
- Bajaj, K.; Adhikary, T.; Gill, P.P.S.; Kumar, A. Edible Coatings Enriched with Plant-Based Extracts Preserve Postharvest Quality of Fruits: A Review. Prog. Org. Coat. 2023, 182, 107669. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of Postharvest Preservation Methods on Nutritional Value and Bioactive Properties of Mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Yildirim-Yalcin, M.; Tornuk, F.; Toker, O.S. Recent Advances in the Improvement of Carboxymethyl Cellulose-Based Edible Films. Trends Food Sci. Technol. 2022, 129, 179–193. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Tomić, M.; Gordić, M.; Nešić, A.; Dimitrijević, S. Response Surface Methodology for Optimisation of Edible Coatings Based on Dextran from Leuconostoc mesenteroides T3. Carbohydr. Polym. 2018, 184, 207–213. [Google Scholar] [CrossRef]
- Gomes, D.; Batista-Silva, J.P.; Sousa, A.; Passarinha, L.A. Progress and Opportunities in Gellan Gum-Based Materials: A Review of Preparation, Characterization and Emerging Applications. Carbohydr. Polym. 2023, 311, 120782. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Salem, A.; Nasri, R.; Nasri, M.; Jridi, M. Food Applications of Bioactive Marine Gelatin Films. Curr. Opin. Food Sci. 2022, 43, 206–215. [Google Scholar] [CrossRef]
- Gagliarini, N.; Diosma, G.; Garrote, G.L.; Abraham, A.G.; Piermaria, J. Whey Protein-Kefiran Films as Driver of Probiotics to the Gut. LWT 2019, 105, 321–328. [Google Scholar] [CrossRef]
- Oyom, W.; Zhang, Z.; Bi, Y.; Tahergorabi, R. Application of Starch-Based Coatings Incorporated with Antimicrobial Agents for Preservation of Fruits and Vegetables: A Review. Prog. Org. Coat. 2022, 166, 106800. [Google Scholar] [CrossRef]
- Mukherjee, K.; Dutta, P.; Badwaik, H.R.; Saha, A.; Das, A.; Giri, T.K. Food Industry Applications of Tara Gum and Its Modified Forms. Food Hydrocoll. Health 2023, 3, 100107. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, L.; Lyu, F.; Ding, Y. The Complex of Whey Protein and Pectin: Interations, Functional Proprerties and Applications in Food Colloidal Systems–A Review. Colloids Surf. B. Biointerfaces 2022, 210, 112253. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Nezafat, N.; Shafiei, N. Chapter 3-Polysaccharide biopolymer chemistry. In Biopoly-mer-Based Metal Nanoparticle Chemistry for Sustainable Applications Volume 1: Classification, Properties and Synthesis; Nasrollahzadeh, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 45–105. [Google Scholar] [CrossRef]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Sena Neto, A.R.; Pinheiro, A.C.M.; Mattoso, L.H.C.; Martins, M.A. Development and Physical-Chemical Properties of Pectin Film Reinforced with Spent Coffee Grounds by Continuous Casting. Carbohydr. Polym. 2019, 210, 92–99. [Google Scholar] [CrossRef]
- Abdin, M.; El-Beltagy, A.E.; El-sayed, M.E.; Naeem, M.A. Production and Characterization of Sodium Alginate/Gum Arabic Based Films Enriched with Syzygium cumini Seeds Extracts for Food Application. J. Polym. Environ. 2022, 30, 1615–1626. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Yang, F.; Wang, T.; Ni, M.; Chen, Y.; Yang, F.; Huang, D.; Fu, C.; Wang, S. Preparation and Characterization of Chitosan-Based Ternary Blend Edible Films with Efficient Antimicrobial Activities for Food Packaging Applications. J. Food Sci. 2019, 84, 1411–1419. [Google Scholar] [CrossRef]
- López-Ortiz, A.; Pacheco Pineda, I.Y.; Méndez-Lagunas, L.L.; Balbuena Ortega, A.; Guerrero Martínez, L.; Pérez-Orozco, J.P.; del Río, J.A.; Nair, P.K. Optical and Thermal Properties of Edible Coatings for Application in Solar Drying. Sci. Rep. 2021, 11, 10051. [Google Scholar] [CrossRef]
- Liang, C.; Jia, M.; Tian, D.; Tang, Y.; Ju, W.; Ding, S.; Tian, L.; Ren, X.; Wang, X. Edible Sturgeon Skin Gelatine Films: Tensile Strength and UV Light-Barrier as Enhanced by Blending with Esculine. J Funct. Foods 2017, 37, 219–228. [Google Scholar] [CrossRef]
- Otálora González, C.M.; Schelegueda, L.I.; Pizones Ruiz-Henestrosa, V.M.; Campos, C.A.; Basanta, M.F.; Gerschenson, L.N. Cassava Starch Films with Anthocyanins and Betalains from Agroindustrial By-Products: Their Use for Intelligent Label Development. Foods 2022, 11, 3361. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Heng, L.Y.; Salam, F.; Zaid, M.H.M.; Hanifah, S.A. A Colorimetric Ph Sensor Based on Clitoria Sp and Brassica Sp for Monitoring of Food Spoilage Using Chromametry. Sensors 2019, 19, 4813. [Google Scholar] [CrossRef]
- Ajayi, E.I.; Molehin, O.R.; Ajayi, O.O.; Adeloju, E.O.; Oladele, J.O. Chapter 28-Application of chitosan-coated foods, fruits and vegetables on inflammation in metabesity. In Next Generation Nanochitosan; Adetunji, C., Hefft, D., Jeevanandam, J., Danquah, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 431–446. [Google Scholar] [CrossRef]
- Rani, P.; Yu, X.; Liu, H.; Li, K.; He, Y.; Tian, H.; Kumar, R. Material, antibacterial and anticancer properties of natural polyphenols incorporated soy protein isolate: A review. Eur. Polym. J. 2021, 152, 110494. [Google Scholar] [CrossRef]
- Leite, A.C.C.O.; Cerqueira, M.A.; Michelin, M.; Fuciños, P.; Pastrana, L. Antiviral edible coatings and films: A strategy to ensure food safety. Trends Food Sci. Technol. 2023, 138, 551–563. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Leite, C.C.O.; Tomas, A.L.; Reiche, A.; Silva, P.M.; Santos, N.C.; Michelin, M.; Fuciños, P. Edible alginate-based films with anti-SARS-CoV-2 activity. Food Microbiol. 2023, 113, 104251. [Google Scholar] [CrossRef]
- Márquez-Rangel, I.; Cruz, M.; Belmares, R. Agave waste as a source of prebiotic polymers: Technological applications in food and their beneficial health effect. Food Biosci. 2023, 56, 103102. [Google Scholar] [CrossRef]
- López de Lacey, M.; Giménez, B.; Montero, P. Bioaccessibility of green tea polyphenols incorporated into an edible agar film during simulated human digestion. Food Res. Int. 2012, 48, 462–469. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Borriello, S.P.; Hammes, W.P.; Holzapfel, W.; Marteau, P.; Schrezenmeir, J.; Vaara, M.; Valtonen, V. Safety of Probiotics That Contain Lactobacilli or Bifidobacteria. Clin. Infect. Dis. 2003, 36, 775–780. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus Rhamnosus GG in Prebiotic Edible Films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef]
- Wong, C.H.; Mak, I.E.K.; Li, D. Bilayer edible coating with stabilized Lactobacillus plantarum 299v improved the shelf life and safety quality of fresh-cut apple slices. Food Packag. Shelf Life. 2021, 30, 100746. [Google Scholar] [CrossRef]
- Phùng, T.T.T.; Gerometta, M.; Karbowiak, T. Comprehensive approach to the protection and controlled release of extremely oxygen sensitive probiotics using edible polysaccharide-based coatings. Int. J. Biol. Macromol. 2022, 218, 706–719. [Google Scholar] [CrossRef]
- Orozco-Parra, J.; Mejía, C.M.; Villa, C.C. Development of a bioactive synbiotic edible film based on cassava starch, inulin, and Lactobacillus casei. Food Hydrocoll. 2020, 104, 105754. [Google Scholar] [CrossRef]
- EN13432:2000; Packaging–Requirements for Packaging Recoverable through Composting and Biodegradation–Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. European Commission Standard: Brussels, Belgium, 2000; pp. 1–26.
- Vargas, V.H.; Marczak, L.D.F.; Flôres, S.H.; Mercali, G.D. Morphology and functional properties of gelatin-based films modified by UV radiation and bacterial cellulose nanofibers. J Food Process Eng. 2023, 46, e14399. [Google Scholar] [CrossRef]
- Rohadi, T.N.T.; Ridzuan, M.J.M.; Majid, M.S.A.; Sulaiman, M.H. Biodegradability of bioplastic film using different regions of Pennisetum purpureum incorporated with gelatine and chitosan. Int. J. Environ. Sci. Technol. 2023, 20, 10313–10324. [Google Scholar] [CrossRef]
- Thakwani, Y.; Karwa, A.; Kumar, B.G.P.; Purkait, M.K.; Changmai, M. A composite starch-date seeds extract based biodegradable film for food packaging application. Food Biosci. 2023, 54, 102818. [Google Scholar] [CrossRef]
- Frangopoulos, T.; Marinopoulou, A.; Goulas, A.; Likotrafiti, E.; Rhoades, J.; Petridis, D.; Kannidou, E.; Stamelos, A.; Theodoridou, M.; Arampatzidou, A.; et al. Optimizing the Functional Properties of Starch-Based Biodegradable Films. Foods 2023, 12, 2812. [Google Scholar] [CrossRef]
- Kesari, A.K.; Mandava, S.; Munagala, C.K.; Nagar, H.; Aniya, V. DES-ultrasonication processing for cellulose nanofiber and its compounding in biodegradable starch based packaging films through extrusion. Ind. Crops Prod. 2022, 188, 115566. [Google Scholar] [CrossRef]
- Zhao, G.; Lyu, X.; Lee, J.; Cui, X.; Chen, W.N. Biodegradable and transparent cellulose film prepared eco-friendly from durian rind for packaging application. Food Packag. Shelf Life 2019, 21, 100345. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, D.; Zhang, J.; Li, J.; Lai, D.; Lin, S.; Hu, J. Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate. Foods 2022, 11, 586. [Google Scholar] [CrossRef]
- Bandyopadhyaya, S.; Sahaa, N.; Brodnjakb, U.V.; Sáha, P. Bacterial cellulose and guar gum based modified PVP-CMC hydrogel films: Characterized for packaging fresh berries. Food Packag. Shelf Life. 2019, 22, 100402. [Google Scholar] [CrossRef]
- Azevedo, E.S.; Norena, C.P.Z. Upcycling of non-pomace residue of grape juice in the functionalization of polyelectrolyte complexes for biodegradable freshness indicators development. Food Hydrocoll. 2023, 143, 108869. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.; Benítez, J.J.; Porras-Vázquez, J.M.; Tedeschi, G.; Morales, Y.; Fernández-Ortuño, D.; Athanassiou, A.; Guz-man-Puyol, S. Plasticized, greaseproof chitin bioplastics with high transparency and biodegradability. Food Hydrocoll. 2023, 145, 109072. [Google Scholar] [CrossRef]
- Moreno, A.G.; Guzman-Puyol, S.; Domínguez, E.; Benítez, J.J.; Segado, P.; Lauciello, S.; Ceseracciu, L.; Porras-Vázquez, J.M.; Le-on-Reina, L.; Heredia, A.; et al. Pectin-cellulose nanocrystal biocomposites: Tuning of physical properties and biodegradability. Int. J. Biol. Macromol. 2021, 180, 709–717. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benítez, J.J.; Tedeschi, G.; Porras-Vázquez, J.M.; Heredia, A.; Athanassiou, A.; Diego Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-blocking, and high barrier cellulose-based bioplastics with naringin as active food packaging materials. Int. J. Biol. Macromol. 2022, 209, 1985–1994. [Google Scholar] [CrossRef]
- Colak, B.Y.; Peynichou, P.; Galland, S.; Oulahal, N.; Assezat, G.; Prochazka, F.; Degraeve, P. Active biodegradable sodium ca-seinate films manufactured by blown-film extrusion: Effect of thermo-mechanical processing parameters and formulation on lysozyme stability. Ind. Crops Prod. 2015, 72, 142–151. [Google Scholar] [CrossRef]
- Chettri, S.; Sharma, N.; Mohite, M. Edible coatings and films for shelf-life extension of fruit and vegetables. Adv. Biomater. 2023, 154, 213632. [Google Scholar] [CrossRef]
- European Comition. Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, L135, 3–11. [Google Scholar]
- Anadón, A.; Bell, D.; Binderup, M.L.; Bursch, W.; Castle, L.; Crebelli, R.; Engel, K.H.; Franz, R.; Gontard, N.; Haertlé, T.; et al. Guidelines on Submission of a Dossier for Safety Evaluation by the EFSA of Active or Intelligent Substances Present in Active and Intelligent Materials and Articles Intended to Come into Contact with Food. EFSA J. 2009, 1208, 1–10. [Google Scholar]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart Packaging Systems for Food Applications: A Review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Valorisation of Micro/Nanoencapsulated Bioactive Compounds from Plant Sources for Food Applications Towards Sustainability. Foods 2023, 12, 32. [Google Scholar] [CrossRef]
- Jafari, S.M. 1-An Overview of Nanoencapsulation Techniques and Their Classification. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–34. [Google Scholar] [CrossRef]
- Garcia-Ibañez, P.; Moreno, D.A.; Carvajal, M. Nanoencapsulation of Bimi® extracts increases its bioaccessibility after in vitro digestion and evaluation of its activity in hepatocyte metabolism. Food Chem. 2022, 385, 132680. [Google Scholar] [CrossRef]
- Kong, I.; Degraeve, P.; Pui, L.P. Polysaccharide-Based Edible Films Incorporated with Essential Oil Nanoemulsions: Physi-co-Chemical, Mechanical Properties and Its Application in Food Preservation—A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef]

| Biological Source | ||||
|---|---|---|---|---|
| Compound Class | Plant | Macroalga | Animal | Microorganism |
| Polysaccharide | Arabic gum Basil seed gum Cellulose and derivatives Corn fibre gum Dextran Flaxseed gum Guar gum Kefiran Konjac gum Maltodextrin Pectin Persian gum Quince seed gum Starch Tamarind gum Tara gum Tragacanth gum Pullulan | Alginate (brown alga) Agar (red alga) Carrageenan (red alga) Fucoidan (brown alga) Laminarin (brown alga) Ulvan (green alga) | Chitosan | Alginate (Bacteria: Pseudomonas aeruginosa) Cellulose and derivatives Chitosan (fungi) Curdlan Cyclodextrin Gellan gum Levan Pullulan (Aureobasidium pullulans) Xanthan gum |
| Protein | Gluten Quinoa protein Wheat protein Zein | Casein Gelatine Soy protein Whey protein | ||
| Lipid | Candelilla wax Carnauba wax Glyceride | Beeswax Shellac Glyceride | ||
| Other polymers | Polyhydroxyalkanoate (PHA) Poly(β-hydroxybutyrate) (PHB) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) Polylactic acid | |||
| Coating Base, Base Solution Concentration | Biological Source | Incorporated Ingredient/s | Food Application: Food Product, Main Results | Reference/s |
|---|---|---|---|---|
| Alginate, 1–3% | Macroalgae (Macrocystis pyrifera, Kelp) | - | Rose apple cv. Tabtimchan, retarded CI. | [17] |
| Aloe vera and frankincense oil; aloe vera and garlic oil; aloe vera | Green capsicum, excellent inhibition of bacteria and fungi; tomato, mechanical, thermal, and antimicrobial properties, UV shielding. | [18,19,20] | ||
| Limonene; loquat leaf extract | Blackberry, lowered microbial growth; Nanfeng tangerine, extended SL. | [21,22] | ||
| Alginate, 1%/CMC, 1%/starch, 1% | Macroalgae/plant | Grapefruit seed extract | Green chili, enhanced the SL by 25 d. | [23] |
| Alginate, 1%/cellulose (hydroxyethyl), 0.5% | Asparagus waste extract | Strawberry fruit, activity against Penicillium italicumy, reduced color changes and WL, maintained TPC and flavonoid content, and extended the SL. | [24] | |
| Alginate, 0.2–0.5%/chitosan, 0.2–0.5% | Macroalgae/animal | - | Japanese pear fruit, extended the SL. | [25] |
| Arabic gum, 2–10% | Plant | Bergamot pomace extract or bergamot EO; Zataria multiflora Boiss EO | Strawberry, low decay rates, good acceptability by consumers, and retention of ascorbic acid for 14 d; pistachio, high free fatty acid and peroxide values. | [26,27] |
| Arabic gum, 10%/CMC, 0.5% | - | Tomato, extension of ripening phase, delaying senescence, and increasing acceptability for longer time. | [28] | |
| Carrageenan (k), 0.2%/chitosan, 0.75% | Macroalgae/animal | - | Dragon fruit, maintained freshness and bract color, retained chlorophyll content and fruit eating quality. | [29] |
| Carrageenan, 0.5%/Arabic gum, 3%/xanthan gum, 0.1% | Macroalgae/plant/bacterial | Lemon grass EO | Strawberry, inhibited psychrophilic bacteria, yeast, and mold, retained quality up to 12 d under refrigeration. | [30] |
| Casein, 0.5% | Animal | Gliadin nanoparticles; methyl jasmonate | Cherry tomato, controlled black rot, alleviated CI during long-term cold storage. | [31,32] |
| Cellulose, 0.6–0.8% | Bacterial (Gluconacetobacter xylin) | Chia seed mucilage | Strawberry, controlled PPO and POD. | [33] |
| Cellulose (CMC), 1–1.5% | Plant | - Cardamom oil; Morus alba root extract | Strawberry, increased the SL better than pectin, tragacanth and persian gums (efficiency in this order); tomato, prevented microbial spoilage; banana, controlled colour, PPO, and BI. | [13,34,35] |
| Cellulose (CMC), 2–10%/pectin, 2–10% | Plant (pectin from banana peel) | - | Tomato, increased SL in cold storage; fruits and vegetables, prevented microbial decay and enzymatic/biochemical, physical/textural changes. | [36,37] |
| Cellulose/chitin/chitosan (1:1:1) | Plant (Miscanthus floridulus straw)/animal (crab shells) | Strawberry, decreased WL and color changes. | [38] | |
| Cellulose, 1%/chitosan, 1.5% | Curcumin | Kiwifruit, reduced WL, firmness loss, and microbial growth for 10 d at 10 °C. | [39] | |
| Cellulose (hydroxypropyl methyl), 1%/carnauba wax, 9–18% | Plant | Ginger EO | Papaya, reduced WL, color development, and slowed ripening. | [40] |
| Cellulose (hydroxypropyl methyl), 5%/beeswax, 10–40% | Plant/animal | - | Mango, increased the SL by 6 d. | [41] |
| Cellulose (nanofiber), 0.8%/zein, 0.8% | Plant (softwood Kraft pulp) | Beeswax or camellia wax emulsion | n.a. | [42] |
| Cellulose, 4–16%/pectin, 1% | Bacterial/plant (citrus peel) | Blackberry pomace | n.a. | [43] |
| Plant (citrus fruit) | p-coumaric acid | Fresh-cut peach, inhibited the browning process within 8 h. | [44] | |
| Cellulose, 0.5%/starch, 4–5% | Basil EO | Mandarin, increased the SL in 12 d at 25 °C. | [45] | |
| Cellulose, 0.5–1.5%/carnauba wax, 0.5–1.5% | - | Pomegranate, extended the SL to 150 d. | [46] | |
| Chitosan, 0.05–2% | Animal (crab shells; shells of Litopenaeus vannamei; crustacean shells) | Caraway oil; canola oil, ginger extract; grapeseed EO, sea buckthorn EO; mixed plant extract of moringa + eucalyptus + marigold; oleic acid; pomelo extract; propolis extract; tea polyphenols; tea seed oil; thymol; thyme oil; Torreya grandis seed EO | Banana: reduced WL, firmness loss, TSS, and TA, maintained color, increased AA, and inhibited microbial growth. Walnuts, reduced A. flavus incidence and spores. Organic strawberries and apples, SL extension. Pitaya, prevented fungi for 15 d and maintained quality parameters. Lychee, inhibited fungal decay and improved storability. Fig, aflatoxin production < 20 ppb and acceptable sensory quality. Broccoli, improved sensory quality and nutraceutical value. Japanese pear, activity against B. cinerea. Grape, improved firmness, AA, anthocyanin, and sensory attributes, extending the SL. Mango, controlled anthracnose better than fungicides. Loquat, reduced WL and decay index and increased TSSs and ascorbic acid. | [47,48,49,50,51,52,53,54,55,56,57] |
| Chitosan, 0.5–2.5%/gelatine, 1–5% | - Black tea extract; lemongrass EO; and β-cyclodextrin | Red guava, delayed ripening, reduced WL and lipid oxidation, maintained color and firmness, and preserved for at least 8 d; papaya, suppressed microbial growth, increased the pH, TSSs, TA, AA, and total carotenoids during storage due to substances in the edible layer; cherry tomato, very effective against fungi for 20 d. | [58,59,60] | |
| Chitosan, 1–1.5%/guar gum, 0.3–25% | Animal/plant (citrus peel) | - | Kinnow fruits, extended the SL up to 25 d at room storage. | [61] |
| Chitosan, 1%/Arabic gum, 10% | Cleistocalyx operculatus extracts and natamycin | Banana, 21 d of storage at room conditions; black Périgord truffles, can affect volatile organic compounds and bacteria implicated in preservation. | [62,63] | |
| Curdlan, 1%/konjac gum, 1% | Bacterial/plant | - | Cherry tomato, reduced WL, decay loss, firmness loss, TSSs, total acid, and volatile compound contents. | [64] |
| Fucoidan, 1–5% | Algae | Mango fruit, extended the SL. | [65] | |
| Gelatine, 1–2% | Animal (fish skin) | - Almond gum | Peanut, binary system of lignin and gelatine favored quality control; tomato, did not affect the pH and colour indices, delayed changes in firmness, lycopene content, WL, and decay. | [66,67] |
| Gelatine, 14.5%/starch, 9.20% | Animal (poultry waste)/plant (lotus)/plant (cassava) | - | Cherry tomato, maintained firmness and the pH and reduced WL during 15 d of storage. | [68] |
| Banana, delayed respiratory peak by 4 d. | [69] | |||
| Guar gum, 1–2.5% | Plant | Castor oil | Mango, increased TPCs and AA and promoted SL extension. | [70] |
| Guar gum, 2.5%/starch, 2.5% | - | Cut apple, storage quality in terms of microbial growth, pH, color, and WL. | [71] | |
| Maltodextrin, 4%/pectin, 6% | Sodium chloride | Starfruit, extended the SL and maintained physicochemical characteristics for 14 d. | [72] | |
| Pectin, 0.5–3.5% | Carvacrol/2-hydroxypropyl-β-cyclodextrin | Strawberry, improved the SL, reduced WL, decay, and preserved nutritional ingredients; n.a. | [13,73] | |
| Plant (orange peels) | Lemon EO or Lemon EO and reuterin from Lactobacillus reuteri | Strawberry, avoid fungal spoilage without quality reduction. | [74] | |
| Pectin, 1.5–3%/beeswax, 10% | Plant/animal | Artocarpus heterophyllus leaf extract | Tomato, controlled Alternaria spp., improved the SL. | [75] |
| Persian gum, 4% | Plant | - | Strawberry, reduced WL and decay, preserved nutritional ingredients at 4 °C. | [13] |
| Shellac, 1–20% | Animal | - Juglone and tannic acid | n.a. Lime, delayed color changes, reduced chlorophyll degradation, affected TA reduction, and enhanced the accumulation of total ascorbic acid and hydrogen peroxide; Wichita pecans, potentially delayed oxidation, maintaining quality in long-term refrigerated storage; mango, SL extension to 10 d, maintaining firmness and WL, reducing browning, lipid peroxidation, preserved aromatic volatiles, and antifungal activity. | [76,77,78,79] |
| Shellac, 10%/zein, 5% | Animal/plant | Thymol | Fresh-cut cantaloupe, efficient encapsulation with high antioxidant and antimicrobial activities. | [80] |
| Starch, 2–10% | - | Strawberry, extended the ripening process by up to 18 d at 20 °C. | [81] | |
| Plant (lima bean) | - | Sapota fruit, potential coating material compared to lima bean pod starch. | [82] | |
| Plant (yam bean) | Agarwood Aetoxylon bouya EO/calcium | Strawberry, maintained quality during storage. | [83] | |
| Starch, 2–5%/beeswax, 33% | Plant/animal | Eichhornia crassipes | Fresh banana, strawberry, and fresh-cut apple, magnificent color and freshness preservation by reducing oxidation, preventing WL. | [84] |
| Starch, 4–5%/cellulose, 0.5% | Plant | Basil EO | Mandarin, promising for extending the SL. | [45] |
| Tragacanth gum, 0.6–1.5% | - | Strawberry, reduced WL and decay and preserved nutritional compounds during storage. | [13] | |
| Wax (bees), 1–10% | Animal | Coconut oil and salicylic acid | Lemon, combined with MAP, maintained quality and shiny green color for 8 weeks; pears, prevented fruit softening. | [85,86] |
| Wax (bees), % n.a/zein, 0.1% | Animal/plant | Nisin | Nectarine and apple, accelerated decline of L. monocytogenes, did not impact the survival and growth of molds and yeasts on nectarine, but performed comparably to wax on apples. | [87] |
| Wax (carnauba), 1–18% | Syzigium aromaticum and Mentha spicata EO; candle soot; oleic acid; polylactic acid; and orange oil | Papaya, can act as antimicrobial; n.a. Fresh tomatoes: highest instrumental gloss and were preferred by consumers; n.a. Salacca, maintained quality and provided moderate organoleptic approval. | [88,89,90,91,92] | |
| Whey protein, 5–15% | Animal (by-product of the cheese process) | - Mango peel extract | Peeled garlic cloves: extended the SL up to 10 d at 15 °C; Fresh-cut broccoli, improved sensory evaluation and reduced total fungi and bacterial counts. | [93,94] |
| Whey protein, 5%/xanthan gum, 1% | Animal/bacterial | Clove oil | Tomato, delayed senescence and maintained firmness. | [95] |
| Whey protein, 15%/zein, 20% | Animal/plant | - | Peeled garlic cloves, extended the SL up to 10 (whey protein) and 15 (zein) d at 15 °C. | [93] |
| Zein, 0.1–20% | Plant | - | Over-peeled garlic cloves, extended the SL up to 15 d at 15 °C. | [93] |
| Plant (corn) | Nisin | Nectarine and apple, promising for mitigating L.monocytogenes contamination. | [87] |
| Film Base, Base Solution Concentration | Biological Source | Incorporated Ingredient/s | Food Application: Food Product, Main Results | Reference/s |
|---|---|---|---|---|
| Agar, 1.1%/Arabic gum, 0.9%/konjac gum, 0.4% | Red algae/plant | Coconut oil | Cucumber, lower WL and firmness reduction at 7 °C. | [96] |
| Agar, 2%/maltodextrin, 2%/beeswax, 0.4–20% | Red algae/plant/animal | n.a. | [97] | |
| Alginate, 1–3% | Macroalgae | Tannic acid; probiotic Bacillus coagulans/Vitis vinifera leaf extract (VVLE) | Activity against E. coli; probiotics in the film exhibited high viability during simulated digestion and showed antimicrobial and antioxidant activities; VVLE films pre-treated through ultrasonication had the highest TPC, AA, and antimicrobial properties. | [98,99,100] |
| Alginate, 0–3%/carrageenan, 0–3%/shellac, % n.a. | Macroalgae/animal | Cellulose nanocrystals | Cherry tomato, excellent film properties to extend the SL; shellac resulted in lower WL. | [101] |
| Alginate, 1.5%/cellulose, 0.5%/beeswax, 1–10% | Macroalgae/plant/animal | Anthocyanin | n.a. | [102] |
| Alginate, 2.5%/Arabic gum, 1% | Macroalgae/plant | Natamycin | Sweet potatoes, slowed down physiological and quality changes, with good quality after 120 days. | [103] |
| Alginate, 2%/konjac gum, 5% | Reuterin | Inhibition of bacteria, B. cereus, C. perfringens, and P. aeruginosa, and fungi, F. oxysporum, A. alternata, C. gloeosporioides, and P. digitatum. | [104] | |
| Alginate, 3%/tragacanth gum, 2–14% | Macroalgae/plant | Aloe vera | n.a. | [105] |
| Alginate, 2%/wheat protein, 4–8% | - | n.a | [106] | |
| Carrageenan (ι-), 0.5–2%/starch, 2–3.5% | Macroalgae/plant | - | n.a. | [107] |
| Carrageenan, 1%/whey protein, 5% | Macroalgae/animal | Probiotics (Lactobacillus acidophilus, Lactobacillus plantarum, and mixed culture) | Probiotic bacteria significantly influenced film water vapor permeability and color. | [108] |
| Casein, 6%/basil seed gum, 1% | Animal (bees)/plant (candelilla and carnauba) | Guar gum/gelatine-based nanogel containing lemon peel EO | Good antioxidant properties, inhibitory effect against E. coli and S. aureus, and no toxicity for endothelial cells line for 72 h. | [109] |
| Casein, 2–12%/pectin, 0.5–5% | Animal/plant | Egg albumin; pea protein isolate | [110,111] | |
| Casein, 5%/starch, 5% | Pomegranate peel extract | AA, antibacterial effect against E. coli and S. aureus, slow release of bioactives in hydroalcoholic medium. | [112] | |
| Cellulose (hydroxypropyl methyl), 1%/curdlan, 1% | Plant/bacterial | Oleic acid | n.a. | [113] |
| Cellulose (hydroxypropyl methyl), 4%/tamarind gum, 13.5%/zein, 8% | Plant (rice husk) | - | Cherry tomato, increased the SL, good UV barrier, transparency, and antibacterial and antioxidant activities. | [114] |
| Cellulose (methyl), 2%/zein, 2% | Oleic acid/thymol | Effective against E. coli and S. aureus. Incorporation of zein reduced water vapor permeability and solubility. | [115] | |
| Cellulose, 2%/soy protein, 3% | - | n.a. | [116] | |
| Cellulose, 1.5%/starch, 5% | Thymol | Antibacterial activity against E. coli. | [117] | |
| Chitosan, 1–1.5% | Animal (crustacean shells of shrimps and fish skin) | tea tree EO | A.A. and antimicrobial properties against L. monocytogenes (tea tree EO 1.5%), transparency, and good UV barrier properties. | [118] |
| Chitosan, 1%/konjac gum, 0.3%/tragacanth gum, 0.7% | Animal/plant (acorn) | Tannic acid and ε-polylysine | n.a. | [119] |
| Chitosan, 1%/tamarind gum, 1–4% | Xyloglucan | Intense antimicrobial activity. | [120] | |
| Chitosan/ulvan % n.a. | - | Ulvan extract increased physicochemical properties and AA. | [121] | |
| Gelatine, 0.2%/Persian gum, 0.2% | Animal (scales and fins of Cyprinus carpio: bovine)/plant | - | n.a. | [122] |
| Gelatine, 3%/pectin, 3%/starch, 2% | Animal/plant | - | Antioxidant and antibacterial activities against E. coli and S. aureus. | [123] |
| Gellan gum, 1%/konjac gum, 0–4% | Bacterial/plant | Dihydromyricetin | Enhanced antioxidant and antimicrobial activities | [124] |
| Gellan gum, 0.8%/levan, 0.8% | Bacterial | - | n.a. | [125] |
| Gellan gum, 0–3%/quince seed gum, 0–3%/starch potato, 0–3% | Bacterial/plant | n.a. | [126] | |
| Gluten, 3–7%/guar gum, 1–2% | Plant | - | n.a. | [127] |
| Persian gum, 2% | Plant | Sunflower oil | n.a. | [128] |
| Starch, 3–6% | Plant (corn) | - | N.a. | [129] |
| Plant (pea) | Araçá extract (Psidium myrtoides) | Inhibits the growth of S. aureus | [130] | |
| Plant (rye) | - - | n.a. | [131] | |
| Plant (sago) | n.a. | [132] | ||
| Starch/polylactic acid % n.a. | Plant (cassava)/microorganism | Ferulic acid/cinnamic acid | Growth inhibition of E. coli, L. innocua and Listeria. | [133] |
| Starch, 1–5%/zein, 0.5–2% | Plant (tapioca) | Natamycin/nisin | n.a. | [134] |
| Wax, 0.1–0.3%/whey protein, 1.15–1.35% | Plant (candelilla)/animal | Tarbush polyphenols | n.a. | [135] |
| Whey protein, 5% | Animal | Lepidium perfoliatum gum | n.a. | [136] |
| Zein, 1% | Plant | Catechin and cyclodextrin | High antioxidant capacity | [137] |
| Film/Coating Base, Base Solution Concentration | Biological Source | Incorporated Ingredient/s | Food Application: Food, Main Results | Reference |
|---|---|---|---|---|
| Alginate, 0.5%/pectin, 0.6%/xanthan gum, 0.4% | Macroalgae/plant (citrus)/bacterial | - | Fresh-cut potato, excellent preservation, coating with better effect than film heat sealing. | [138] |
| Agar/cellulose/gelatine/gellan/k-carrageenan/tamarind gum % n.a. | Red algae/plant/animal | - | Strawberry, reduced WL, PPO and POD activities, maintained firmness, ASA, TSS, and TA. | [16] |
| Casein, 2.5%/chitosan, 2% | Animal | Origanum vulgare L. essential oil | Cherry tomato, fungal growth inhibited for 28 days at 4 °C. | [139] |
| Cellulose (ligno), 2%/wheat gluten, 10% | Plant | - | Cherry, litchi, and waxberry, excellent antimicrobial properties and UV blocking. | [140] |
| Chitosan, 2%/levan, 2%/pullulan, 2% | Animal/bacterial (halotolerant Bacillus sp)/yeast | ε-polylysine | Strong inhibitory effect on two typical food-borne pathogens; WL, firmness, and TSSs of coated strawberries tended to decrease. | [141] |
| Corn fiber gum/chitosan (3:1) | Plant/animal | Carvacrol | Tomato and fresh-cut apple, reduced Listeria, E. coli, for up to 7 and 21 days, respectively, at 4 °C. | [142] |
| Corn fiber gum/whey protein (1:3) | Plant/animal | |||
| Gellan gum, 2% | Bacterial | Cranberry extract/Lactococcus lactis | Fresh-cut potato and apple, probiotic film for optimal preservation; enhanced antibacterial and antioxidant activities. | [143] |
| Starch, 5% | Plant | Carvacrol/thymol | Mango and papaya, reduced the incidence of the fungus responsible for anthracnose. | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Recent Highlights in Sustainable Bio-Based Edible Films and Coatings for Fruit and Vegetable Applications. Foods 2024, 13, 318. https://doi.org/10.3390/foods13020318
Martins VFR, Pintado ME, Morais RMSC, Morais AMMB. Recent Highlights in Sustainable Bio-Based Edible Films and Coatings for Fruit and Vegetable Applications. Foods. 2024; 13(2):318. https://doi.org/10.3390/foods13020318
Chicago/Turabian StyleMartins, Valter F. R., Manuela E. Pintado, Rui M. S. C. Morais, and Alcina M. M. B. Morais. 2024. "Recent Highlights in Sustainable Bio-Based Edible Films and Coatings for Fruit and Vegetable Applications" Foods 13, no. 2: 318. https://doi.org/10.3390/foods13020318
APA StyleMartins, V. F. R., Pintado, M. E., Morais, R. M. S. C., & Morais, A. M. M. B. (2024). Recent Highlights in Sustainable Bio-Based Edible Films and Coatings for Fruit and Vegetable Applications. Foods, 13(2), 318. https://doi.org/10.3390/foods13020318