In Vitro Hypoglycemic Activities of Lactobacilli and Bifidobacterium Strains from Healthy Children’s Sources and Their Effect on Stimulating GLP-1 Secretion in STC-1 Cells
Abstract
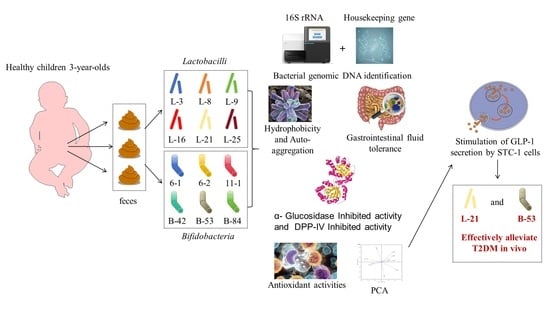
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Selection and Collection
2.3. Strains and Cell Culture
2.4. Isolation and Identification of Strains
2.4.1. Isolation of Strains
2.4.2. Bacterial Genomic DNA Extraction and Identification
2.4.3. Phylogenetic Tree Construction
2.5. Preparation of Sample
2.5.1. Bacterial Cell Resuspension
2.5.2. Cell-Free Supernatants and Extracts
2.6. Determination of Hydrophobicity
2.7. Auto-Aggregation Measurement
2.8. Gastrointestinal Tolerance Test
2.9. Inhibitory Activity Assay of α-Glucosidase and DPP-IV
2.9.1. α-Glucosidase Inhibitory Activity
- A1: PNPG + sample + α-Glucosidase + Na2CO3.
- A2: PNPG + sample + PBS (0.1 mol/L, pH 6.8) + Na2CO3.
- A3: PNPG + PBS (0.1 mol/L, pH 6.8) + α-Glucosidase + Na2CO3.
- A4: PNPG + PBS (0.1 mol/L, pH 6.8) + PBS(0.1 mol/L, pH 6.8) + Na2CO3.
2.9.2. DPP-IV Inhibitory Activity
- A1 (sample): Gly-pro-p-nitroanilide + sample + DPP-IV + sodium acetate buffer.
- A2 (sample blank): Gly-pro-p-nitroanilide + sample + Tris-HCl buffer (100 mM, pH 8.0) + sodium acetate buffer.
- A3 (positive control): Gly-pro-p-nitroanilide + Tris-HCl buffer (100 mM, pH 8.0) + DPP-IV + sodium acetate buffer.
- A4 (negative control): Gly-pro-p-nitroanilide + Tris-HCl buffer (100 mM, pH 8.0) + Tris-HCl buffer (100 mM, pH 8.0) + sodium acetate buffer.
2.10. Antioxidant Activities
2.10.1. Reducing Activity
2.10.2. DPPH Free Radical-Scavenging Activity
2.10.3. Hydroxyl Radical-Scavenging Activity
2.10.4. Superoxide Anion Radical-Scavenging Activity
2.10.5. Lipid Peroxidation Inhibiting Capacity
2.10.6. Principal Component Analysis (PCA)
2.11. GLP-1 Secretion Assay
2.12. qRT-PCR
2.13. Statistical Analysis
3. Results
3.1. Characterization of Isolated Strains
3.2. Hydrophobicity of Isolated Strains
3.3. Auto-Aggregation of Isolated Strains
3.4. Gastrointestinal Fluid Tolerance
3.5. Hypoglycemic Potential of Isolated Strains
3.5.1. α-Glucosidase Inhibitory Activity
3.5.2. DPP-IV Inhibitory Activity
3.6. Antioxidative Activity of Isolated Strains
3.6.1. Reducing Activity
3.6.2. DPPH Radical-Scavenging Activity
3.6.3. Hydroxyl Radical-Scavenging Ability
3.6.4. Superoxide Anion Radical-Scavenging Ability
3.6.5. Lipid Peroxidation Inhibition Capacity
3.6.6. Principal Component Analysis
3.7. Stimulation GLP-1 Secretion of STC-1 Cells by Lactobacilli and Bifidobacterium Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, W.; Song, X.; Li, X.; Jia, L.; Zhang, C. Structural characterization of Hericium erinaceus polysaccharides and the mechanism of anti-T2DM by modulating the gut microbiota and metabolites. Int. J. Biol. Macromol. 2023, 242, 125165. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Guan, L.; He, X.; Zhang, H.; Zhang, J.; Li, J.; Zhong, D.; Jin, R. A systematic review and meta-analysis of the prevalence and risk factors of depression in type 2 diabetes patients in China. Front. Med. 2022, 9, 759499. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Hussain, M.E. Obesity and diabetes: An update. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 73–79. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, H.; Xu, K.; Du, Y.; Liu, J.; Wang, J.; Jiang, Y. Fecal metabolomics reveals the positive effect of ethanol extract of propolis on T2DM mice. Food Sci. Hum. Wellness 2023, 12, 161–172. [Google Scholar] [CrossRef]
- Wang, D.; Zhai, J.-X.; Liu, D.-W. Serum folate, vitamin B12 levels and diabetic peripheral neuropathy in type 2 diabetes: A meta-analysis. Mol. Cell. Endocrinol. 2017, 443, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Luong, A.; Tawfik, A.N.; Islamoglu, H.; Gobriel, H.S.; Ali, N.; Ansari, P.; Shah, R.; Hung, T.; Patel, T.; Henson, B.; et al. Periodontitis and diabetes mellitus co-morbidity: A molecular dialogue. J. Oral Biosci. 2021, 63, 360–369. [Google Scholar] [CrossRef]
- Soltanieh, S.; Salavatizadeh, M.; Poustchi, H.; Yari, Z.; Mansour, A.; Khamseh, M.E.; Malek, M.; Alaei-Shahmiri, F.; Hekmatdoost, A. The association of dietary inflammatory index (DII) and central obesity with non-alcoholic fatty liver disease (NAFLD) in people with diabetes (T2DM). Heliyon 2023, 9, e13983. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, X.-N.; Li, J.; Lu, J.; Wu, J.; Zhu, W.-F.; Qin, P.; Xu, N.-Z.; Zhang, Q. Increased plasma miR-146a levels are associated with subclinical atherosclerosis in newly diagnosed type 2 diabetes mellitus. J. Diabetes Its Complicat. 2020, 34, 107725. [Google Scholar] [CrossRef]
- Yang, L.; Sakandar, H.A.; Sun, Z.; Zhang, H. Recent advances of intestinal microbiota transmission from mother to infant. J. Funct. Foods 2021, 87, 104719. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Ravel, J. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar]
- Van De Wijgert, J.H.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, X.; Chen, H.; Sun, Y.; Yang, L.; Ma, Y.; Chan, E.C.Y. Antidiabetic effects of multi-species probiotic and its fermented milk in mice via restoring gut microbiota and intestinal barrier. Food Biosci. 2022, 47, 101619. [Google Scholar] [CrossRef]
- Pegah, A.; Abbasi-Oshaghi, E.; Khodadadi, I.; Mirzaei, F.; Tayebinia, H. Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats. Metab. Open 2021, 10, 100093. [Google Scholar] [CrossRef] [PubMed]
- Miguéns-Gómez, A.; Casanova-Martí, À.; Blay, M.T.; Terra, X.; Beltrán-Debón, R.; Rodríguez-Gallego, E.; Ardévol, A.; Pinent, M. Glucagon-like peptide-1 regulation by food proteins and protein hydrolysates. Nutr. Res. Rev. 2021, 34, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, A.L.; Green, B.D. The bioactive effects of casein proteins on enteroendocrine cell health, proliferation and incretin hormone secretion. Food Chem. 2016, 211, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Power-Grant, O.; Bruen, C.; Brennan, L.; Giblin, L.; Jakeman, P.; FitzGerald, R.J. In vitro bioactive properties of intact and enzymatically hydrolysed whey protein: Targeting the enteroinsular axis. Food Funct. 2015, 6, 972–980. [Google Scholar] [CrossRef]
- Dai, T.; Chen, J.; McClements, D.J.; Li, T.; Liu, C. Investigation the interaction between procyanidin dimer and α-glucosidase: Spectroscopic analyses and molecular docking simulation. Int. J. Biol. Macromol. 2019, 130, 315–322. [Google Scholar] [CrossRef]
- Nag, S.; Mandal, S.; Majumdar, T.; Mukhopadhyay, S.; Kundu, R. FFA-Fetuin-A regulates DPP-IV expression in pancreatic beta cells through TLR4-NFkB pathway. Biochem. Biophys. Res. Commun. 2023, 647, 55–61. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Alarcón, M.; Díaz-Maroto, I.J.; Pérez-Coello, M.S.; Soriano, A. Rapid and non-invasive estimation of total polyphenol content and antioxidant activity of natural corks by NIR spectroscopy and multivariate analysis. Food Packag. Shelf Life 2023, 38, 101099. [Google Scholar] [CrossRef]
- Naser, S.M.; Dawyndt, P.; Hoste, B.; Gevers, D.; Vandemeulebroecke, K.; Cleenwerck, I.; Vancanneyt, M.; Swings, J. Identification of lactobacilli by pheS and rpoA gene sequence analyses. Int. J. Syst. Evol. Microbiol. 2007, 57, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Canchaya, C.; Del Casale, A.; Dellaglio, F.; Neviani, E.; Fitzgerald, G.F.; van Sinderen, D. Analysis of bifidobacterial evolution using a multilocus approach. Int. J. Syst. Evol. Microbiol. 2006, 56, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Effect of dairy probiotic combinations on in vitro gastrointestinal tolerance, intestinal epithelial cell adhesion and cytokine secretion. J. Funct. Foods 2014, 8, 18–25. [Google Scholar] [CrossRef]
- Cui, Y.; Han, X.; Hu, X.; Li, T.; Li, S. Distinctions in structure, rheology, antioxidation, and α-glucosidase inhibitory activity of β-glucans from different species. Int. J. Biol. Macromol. 2023, 253, 127684. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Wang, Y.; Luo, J.; Zhao, Y.; Han, G.; Han, L.; Yu, Q. Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded cowhide collagen. Food Chem. 2023, 405, 134793. [Google Scholar] [CrossRef]
- Mananga, M.-J.; Didier, K.B.; Taptue, C.K.; Fadimatou, B.; Ruth, D.N.; Gilbert, M.M.; Blaise, K.; Michelle, D.; Fokou, E.; Marie Modestine, K.S. Nutritional and antinutritional characteristics of ten red bean cultivars (Phaseolus vulgaris L.) from Cameroon. Int. J. Biochem. Res. Rev. 2021, 30, 1–14. [Google Scholar] [CrossRef]
- Wang, T.; Guo, N.; Wang, S.-X.; Kou, P.; Zhao, C.-J.; Fu, Y.-J. Ultrasound-negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food Bioprod. Process. 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Li, F.; Liu, P. Active polysaccharides from Lentinula edodes and Pleurotus ostreatus by addition of corn straw and xylosma sawdust through solid-state fermentation. Int. J. Biol. Macromol. 2023, 228, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Rwubuzizi, R.; Kim, H.; Holzapfel, W.H.; Todorov, S.D. Beneficial, safety, and antioxidant properties of lactic acid bacteria: A next step in their evaluation as potential probiotics. Heliyon 2023, 9, e15610. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-K.; Chiang, B.-H.; Chen, Y.-S.; Yang, J.-H.; Liu, C.-L. Improving the antioxidant activity of buckwheat (Fagopyrum tataricm Gaertn) sprout with trace element water. Food Chem. 2008, 108, 633–641. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. LWT 2017, 86, 438–446. [Google Scholar] [CrossRef]
- Wu, D.Q.; Ding, X.S.; Zhao, B.; An, Q.; Guo, J.S. The essential role of hydrophobic interaction within extracellular polymeric substances in auto-aggregation of P. stutzeri strain XL-2. Int. Biodeterior. Biodegrad. 2022, 171, 105404. [Google Scholar] [CrossRef]
- Lianza, M.; Poli, F.; Nascimento, A.M.D.; da Silva, A.S.; da Fonseca, T.S.; Toledo, M.V.; Simas, R.C.; Chaves, A.R.; Leitão, G.G.; Leitão, S.G. In vitro α-glucosidase inhibition by Brazilian medicinal plant extracts characterised by ultra-high performance liquid chromatography coupled to mass spectrometry. J. Enzym. Inhib. Med. Chem. 2022, 37, 554–562. [Google Scholar] [CrossRef]
- Cao, X.; Xia, Y.; Liu, D.; He, Y.; Mu, T.; Huo, Y.; Liu, J. Inhibitory effects of Lentinus edodes mycelia polysaccharide on α-glucosidase, glycation activity and high glucose-induced cell damage. Carbohydr. Polym. 2020, 246, 116659. [Google Scholar] [CrossRef]
- Lawson, M.A.; O’neill, I.J.; Kujawska, M.; Javvadi, S.G.; Wijeyesekera, A.; Flegg, Z.; Chalklen, L.; Hall, L.J. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 2020, 14, 635–648. [Google Scholar] [CrossRef]
- Munoz-Quezada, S.; Chenoll, E.; Vieites, J.M.; Genovés, S.; Maldonado, J.; Bermúdez-Brito, M.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F. Isolation, identification and characterisation of three novel probiotic strains (Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036) from the faeces of exclusively breast-fed infants. Br. J. Nutr. 2013, 109, S51–S62. [Google Scholar] [CrossRef]
- Liu, G.; Ren, L.; Song, Z.; Wang, C.; Sun, B. Purification and characteristics of bifidocin A, a novel bacteriocin produced by Bifidobacterium animals BB04 from centenarians’ intestine. Food Control. 2015, 50, 889–895. [Google Scholar] [CrossRef]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of potentially probiotic lactic acid bacteria and bifidobacteria isolated from human colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, J.J.; Zhang, H.X.; Xie, Y.H.; Liu, H.; Ren, J.H.; Ren, F.Z.; Jin, J.H. Bifidobacterium animalis subsp. lactis A12 prevents obesity-associated dyslipidemia by modulating gut microbiota-derived short-chain fatty acid production and energy metabolism in high-fat diet-fed mice. Food Nutr. Res. 2022, 66, 8670. [Google Scholar]
- Zhong, H.; Abdullah; Zhang, Y.P.; Zhao, M.J.; Zhang, J.H.; Zhang, H.; Xi, Y.H.; Cai, H.Y.; Feng, F.Q. Screening of novel potential antidiabetic Lactobacillus plantarum strains based on in vitro and in vivo investigations. LWT Food Sci. Technol. 2021, 139, 110526. [Google Scholar] [CrossRef]
- Saito, K.; Tomita, S.; Nakamura, T. Aggregation of Lactobacillus brevis associated with decrease in pH by glucose fermentation. Biosci. Biotechnol. Biochem. 2019, 83, 1523–1529. [Google Scholar] [CrossRef]
- Marco, M.L.; Tachon, S. Environmental factors influencing the efficacy of probiotic bacteria. Curr. Opin. Biotechnol. 2013, 24, 207–213. [Google Scholar] [CrossRef]
- Su, L.-J.; Liu, Y.-Q.; Liu, H.; Wang, Y.; Li, Y.; Lin, H.-M.; Wang, F.-Q.; Song, A.-D. Linking lignocellulosic dietary patterns with gut microbial Enterotypes of Tsaitermes ampliceps and comparison with Mironasutitermes shangchengensis. Genet. Mol. Res. 2015, 14, 13954–13967. [Google Scholar] [CrossRef]
- Tallawi, M.; Opitz, M.; Lieleg, O. Modulation of the mechanical properties of bacterial biofilms in response to environmental challenges. Biomater. Sci. 2017, 5, 887–900. [Google Scholar] [CrossRef]
- Gunyakti, A.; Asan-Ozusaglam, M. Lactobacillus gasseri from human milk with probiotic potential and some technological properties. LWT 2019, 109, 261–269. [Google Scholar] [CrossRef]
- Rijnaarts, H.H.M.; Norde, W.; Bouwer, E.J.; Lyklema, J.; Zehnder, A.J.B. Bacterial adhesion under static and dynamic conditions. Appl. Environ. Microbiol. 1993, 59, 3255–3265. [Google Scholar] [CrossRef]
- Juntarachot, N.; Sunpaweravong, S.; Kaewdech, A.; Wongsuwanlert, M.; Ruangsri, P.; Pahumunto, N.; Teanpaisan, R. Characterization of adhesion, anti-adhesion, co-aggregation, and hydrophobicity of Helicobacter pylori and probiotic strains. J. Taibah Univ. Med. Sci. 2023, 18, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Mandal, H.; Jariwala, R.; Bagchi, T. Isolation and characterization of lactobacilli from human faeces and indigenous fermented foods for their potential application as probiotics. Can. J. Microbiol. 2016, 62, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Pan, L.; Xu, M.; Wang, X.; Wang, Q.; Han, Y. Probiotic potential of Lactobacillus sakei L-7 in regulating gut microbiota and metabolism. Microbiol. Res. 2023, 274, 127438. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Bera, T.; Mondal, S.; Ganguli, A. A novel probiotic strain of Lactobacillus fermentum TIU19 isolated from Haria beer showing both in vitro antibacterial and antibiofilm properties upon two multi resistant uro-pathogen strains. Curr. Res. Microb. Sci. 2022, 3, 100150. [Google Scholar] [CrossRef]
- Hove, H.; Nørgaard, H.; Mortensen, P.B. Lactic acid bacteria and the human gastrointestinal tract. Eur. J. Clin. Nutr. 1999, 53, 339–350. [Google Scholar] [CrossRef]
- Ramchandran, L.; Shah, N.P. Effect of exopolysaccharides and inulin on the proteolytic, angiotensin-I-converting enzyme-and alpha-glucosidase-inhibitory activities as well as on textural and rheological properties of low-fat yogurt during refrigerated storage. Dairy Sci. Technol. 2009, 89, 583–600. [Google Scholar] [CrossRef]
- Pyclik, M.; Srutkova, D.; Schwarzer, M.; Gorska, S. Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins–their chemical structure and biological attributes. Int. J. Biol. Macromol. 2020, 147, 333–349. [Google Scholar] [CrossRef]
- Kansandee, W.; Moonmangmee, D.; Moonmangmee, S.; Itsaranuwat, P. Characterization and Bifidobacterium sp. growth stimulation of exopolysaccharide produced by Enterococcus faecalis EJRM152 isolated from human breast milk. Carbohydr. Polym. 2019, 206, 102–109. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, J.; Zuo, F.; Yu, R.; Zhang, Y.; Ma, H.; Chen, S. Bifidobacteria possess inhibitory activity against dipeptidyl peptidase-IV. Lett. Appl. Microbiol. 2016, 62, 250–255. [Google Scholar] [CrossRef]
- Aiello, G.; Li, Y.; Boschin, G.; Bollati, C.; Arnoldi, A.; Lammi, C. Chemical and biological characterization of spirulina protein hydrolysates: Focus on ACE and DPP-IV activities modulation. J. Funct. Foods 2019, 63, 103592. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl peptidase IV inhibitory properties of a whey protein hydrolysate: Influence of fractionation, stability to simulated gastrointestinal digestion and food–drug interaction. Int. Dairy J. 2013, 32, 33–39. [Google Scholar] [CrossRef]
- Yoshii, K.; Ogasawara, M.; Wada, J.; Yamamoto, Y.; Inouye, K. Exploration of dipeptidyl-peptidase IV (DPP IV) inhibitors in a low-molecular mass extract of the earthworm Eisenia fetida and identification of the inhibitors as amino acids like methionine, leucine, histidine, and isoleucine—ScienceDirect. Enzym. Microb. Technol. 2020, 137, 109534. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel milk protein hydrolysates generated with trypsin. J. Funct. Foods 2017, 34, 49–58. [Google Scholar] [CrossRef]
- Cai, T.; Wu, H.; Qin, J.; Qiao, J.; Yang, Y.; Wu, Y.; Qiao, D.; Xu, H.; Cao, Y. In vitro evaluation by PCA and AHP of potential antidiabetic properties of lactic acid bacteria isolated from traditional fermented food. LWT Food Sci. Technol. 2019, 115, 108455. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Dong, J.; Shi, J.; Guan, J.; Liu, D.; Liu, F.; Li, B.; Huo, G. Identification, Characterization, and antioxidant potential of Bifidobacterium longum subsp. longum strains isolated from feces of healthy infants. Front. Microbiol. 2021, 12, 756519. [Google Scholar] [CrossRef]
- Grespan, E.; Giorgino, T.; Natali, A.; Ferrannini, E.; Mari, A. Different mechanisms of GIP and GLP-1 action explain their different therapeutic efficacy in type 2 diabetes. Metabolism 2021, 114, 154415. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Larsen, P.; Tang-Christensen, M.; Holst, J.; Ørskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997, 77, 257–270. [Google Scholar] [CrossRef]
- Bell, G.I.; Santerre, R.F.; Mullenbach, G.T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 1983, 302, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.; Mayne, J.; Mbikay, M.; Woulfe, J.; Chrétien, M. Expression of PCSK1 (PC1/3), PCSK2 (PC2) and PCSK3 (furin) in mouse small intestine. Regul. Pept. 2009, 152, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef] [PubMed]








| Strain Name | Gene Name | Primer Sequence (5′ → 3′) |
|---|---|---|
| Primer for 16SrRNA gene amplification | ||
| Lactobacilli | 27F | AGAGTTGATCCTGGCTCAG |
| 1495R | CTACGGCTACCTTGTTAGA | |
| Bifidobacteria | Bif285 | GAGGGTTCGATCTGGCTCAG |
| 261 | AAGGAGGTGATCCAGCCGCA | |
| Primers for housekeeping gene amplification | ||
| Lactobacilli | pheS(21 F) | CAYCCNGCHCGYGAYATGC |
| pheS(23 R) | GCRTGRACCAVCCNGCHCC′ | |
| Bifidobacteria | ClpC(uni) | GATACCCAAGTACATCGAG |
| ClpC(Rev) | CATCCTCATCGTCGAACAGAAC | |
| Gene Name | Primer Sequence (5′~3′) |
|---|---|
| GAPDH (F) | AACTTTGGCATTGTGGAAGG |
| GAPDH (R) | CCCTGTTGCTGTAGCCGTAT |
| Pro-glucagon (F) | AATCTTGCCACCAGGGACTT |
| Pro-glucagon (R) | AGTGACTGGCACGAGATGTT |
| PCSK1 (F) | TGGTGATTACACAGACCAGCG |
| PCSK1 (R) | CTCCAAGGCCAGAGCAAAGA |
| Lactobacilli | Aggregate Score | Bifidobacterium | Aggregate Score |
|---|---|---|---|
| LGG | −1.05 | BB12 | −0.22 |
| L-3 | −0.13 | 6-1 | 0.31 |
| L-8 | −0.24 | 11-1 | 0.09 |
| L-9 | 0.29 | B-53 | 0.54 |
| L-21 | 0.23 | B-84 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Chen, J.; Zhang, Y.; Li, X.; Zhang, N.; Liu, F.; Jiao, Y. In Vitro Hypoglycemic Activities of Lactobacilli and Bifidobacterium Strains from Healthy Children’s Sources and Their Effect on Stimulating GLP-1 Secretion in STC-1 Cells. Foods 2024, 13, 519. https://doi.org/10.3390/foods13040519
Cheng Z, Chen J, Zhang Y, Li X, Zhang N, Liu F, Jiao Y. In Vitro Hypoglycemic Activities of Lactobacilli and Bifidobacterium Strains from Healthy Children’s Sources and Their Effect on Stimulating GLP-1 Secretion in STC-1 Cells. Foods. 2024; 13(4):519. https://doi.org/10.3390/foods13040519
Chicago/Turabian StyleCheng, Zhiliang, Jingru Chen, Yulong Zhang, Xinyi Li, Ning Zhang, Fei Liu, and Yuehua Jiao. 2024. "In Vitro Hypoglycemic Activities of Lactobacilli and Bifidobacterium Strains from Healthy Children’s Sources and Their Effect on Stimulating GLP-1 Secretion in STC-1 Cells" Foods 13, no. 4: 519. https://doi.org/10.3390/foods13040519
APA StyleCheng, Z., Chen, J., Zhang, Y., Li, X., Zhang, N., Liu, F., & Jiao, Y. (2024). In Vitro Hypoglycemic Activities of Lactobacilli and Bifidobacterium Strains from Healthy Children’s Sources and Their Effect on Stimulating GLP-1 Secretion in STC-1 Cells. Foods, 13(4), 519. https://doi.org/10.3390/foods13040519