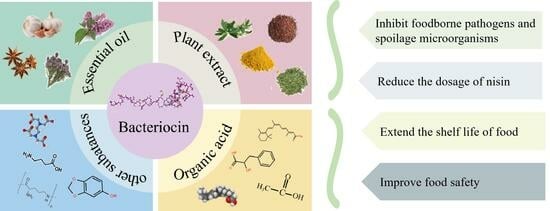
Potential Impact of Combined Inhibition by Bacteriocins and Chemical Substances of Foodborne Pathogenic and Spoilage Bacteria: A Review
Abstract
:1. Introduction
2. Concept, Classification, and Mode of Action of Bacteriocins
2.1. Concept of Bacteriocins
2.2. Classification of Bacteriocins
2.3. Mode of Action of Bacteriocins
3. Combinations of Bacteriocins and Chemical Substances
3.1. Bacteriocins and Essential Oils (EOs)
| Bacteriocins | EO | Food System | Target Microorganisms | Result | References |
|---|---|---|---|---|---|
| Nisin | Perilla frutescense EO | Strawberry | S. aureus; E. coli; S. enteritidis; P. tolaasii | Cytoplasmic efflux, cell lysis and death | [103] |
| Nisin | Carvacrol | Sliced bologna sausage | L. monocytogenes 10403S | Retards bacterial reproduction | [104] |
| Nisin | Carvacrol | Pasteurized milk | S. aureus BNCC 186,335 | Cell membrane damage, nucleic acid, protein leakage | [105] |
| Nisin | Garlic EO | — | L. monocytogenes ATCC 19118 | The cell membrane forms pores | [106] |
| Nisin | Ocimum basilicum, Salvia officinalis and Trachyspermum ammi EOs | — | E. coli O157 (ATCC 25922) | The membrane permeability, proton motility, efflux of amino acids and pH gradient of bacteria are changed | [107] |
| Enteriocin A | Thymus vulgaris EOs | — | L. monocytogenes; E. coli O157:H7 | Inhibition of bacterial growth | [109] |
| Nisin | Clove EO | Pork patties in cold storage | TVC; Psychrotrophs; Enterobacteriaceae; LAB | Inhibit the growth of psychrophilic bacteria, Enterobacteriaceae and LAB in pork samples | [111] |
| Nisin | Oregano EO | Grass carp (Ctenopharyngodon idellus) | TVC | The storage period was prolonged, and the TVC decreased | [113] |
| Nisin | Star anise EO | Ready-to-eat Yao meat products | E. coli | Increase the pH value of pork and volatile salt base total nitrogen, delay the proliferation of E. coli, prolong the shelf life | [114] |
3.2. Bacteriocins and Plant Extracts
| Bacteriocins | Plant Extract | Food System | Target Microorganisms | Result | References |
|---|---|---|---|---|---|
| Nisin | Grape seed extract | Shrimps | L. monocytogenes (stereotype 3a, SSA184) | Protein and nucleic acid leakage | [128] |
| Nisin | Grape seed extract | Shrimps | L. monocytogenes (SSA184, SSA97 and LM10) | The number of L. monocytogenes decreased | [129] |
| Nisin | Grape seed extract | Fresh pork | TVB | The TVB count increased | [130] |
| Nisin | Garlic extract | Milk | L. monocytogenes ATCC 7644; S. Enteritidis SE86; E. coli ATCC 8739; S. aureus ATCC 1901 | Inhibition of bacterial growth | [131] |
| Nisin | Thymoquinone | Sterilized milk | L. monocytogenes ATCC 19115 and ATCC 15313 | Damage membrane integrity, inhibit bacterial growth | [132] |
| Nisin | Green tea extract | Beet leaves | E. coli ATCC 25922 | Inhibit the growth of bacteria and improve the texture and sensory properties of food | [133] |
| Nisin | Laperrinei leave extract | Camel meat | — | Inhibit the rapid increase of thiobarbituric acid value and ferromyoglobin | [134] |
| Nisin | Curcumin | Rainbow trout fillet | LAB | Retards bacterial growth | [48] |
3.3. Bacteriocins and Organic Acids (OAs)
| Bacteriocins | OA | Food System | Target Microorganisms | Result | References |
|---|---|---|---|---|---|
| Nisin | Acetic and propionic acids | Meat and potato | B. subtilis ATCC 6633; S. aureus ATCC 6538; E. coli ATCC 8239 | Inhibit bacterial growth, delay food spoilage | [145] |
| Nisin | Formic acid | Potato | B. subtilis | Inhibit the proliferation of bacteria and color change | [144] |
| Nisin | Free fatty acid | — | L. monocytogenes (ATCC15313 and ATCC19115) | Destroyed the cells in the L. monocytogenes biofilm | [147] |
| Nisin | Lactic acid | Enoki mushrooms | L. monocytogenes (IL-1, ScottA, and IL-1); E. coli O157:H7(ATCC 43889, ATCC 43894, and ATCC 13895) | Inhibition of bacterial proliferation | [146] |
| Nisin | Citric acid | Pasteurized milk | S. aureus (ATCC 29213); L. monocytogenes (ATCC 19115) | Cell surface damage, cell contents (K+, , DNA, RNA) release, inhibit bacterial proliferation | [148] |
| Nisin | Phytic acid | Cold-stored beef | E. coli O157:H7 | The cell biofilm is dissolved | [149] |
3.4. Bacteriocins and Other Substances
| Bacteriocins | Other Substances | Food System | Target Microorganisms | Result | References |
|---|---|---|---|---|---|
| Nisin | Sucrose laurate | Milk beverage | S. aureus ATCC 25923 | Cell wall damage, cell morphology damage | [151] |
| Nisin | Viburnum opulus L. | — | S. aureus NCTC 10788 | DNA, protein leakage | [152] |
| Nisin | EDTA | Pork | TVB; LAB | Retarded bacterial growth and inhibited lipid degradation | [154] |
| Bacteriocins form L. curvatus CRL705 and L. lactis CRL1109 | Na2EDTA | Frozen ground-beef patties | E. coli O157:H7 | Decrease in bacterial population | [156] |
| Nisin | EDTA | Fish fillet | E. coli BCRC 11,634; S. aureus BCRC 10,451 | The antibacterial activity of the membrane was improved | [157] |
| Nisin | γ-Aminobutyric acid | Pork and strawberry | — | Prolong storage time | [158] |
| Nisin | ε-Polylysine | Frankfurter-type sausage | TVC; B. subtilis | Most of the outermost layer of bacterial cells disappeared | [159] |
| Nisin | Chitosan, Tea Polyphenols | Plant-Based Meat | E. coli; S. aureus | Inhibition of bacterial growth | [162] |
| Nisin | Sesamol | Pasteurized milk | L. monocytogenes ATCC 19112 | Surface zeta potential and conductivity rise | [52] |
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nisa, M.; Dar, R.A.; Fomda, B.A.; Nazir, R. Combating food spoilage and pathogenic microbes via bacteriocins: A natural and eco-friendly substitute to antibiotics. Food Control 2023, 149, 109710. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, J.; Ahn, C. Characterization of novel bacteriocin produced by bacteriocinogenic Carnobacterium maltaromaticum isolated from raw milk. Microb. Pathog. 2022, 173, 105872. [Google Scholar] [CrossRef]
- Lei, W.; Hao, L.; You, S.; Yao, H.; Liu, C.; Zhou, H. Partial purification and application of a bacteriocin produced by probiotic Lactococcus lactis C15 isolated from raw milk. LWT-Food Sci. Technol. 2022, 169, 113917. [Google Scholar] [CrossRef]
- Levy, N.e.; Hashiguchi, T.C.O.; Cecchini, M. Food safety policies and their effectiveness to prevent foodborne diseases in catering establishments: A systematic review and meta-analysis. Food Res. Int. 2022, 156, 111076. [Google Scholar] [CrossRef]
- Zanetta, L.D.A.; Hakim, M.P.; Stedefeldt, E.; Rosso, V.V.D.; Cunha, L.M.; Redmond, E.C.; Cunha, D.T.d. Consumer risk perceptions concerning different consequences of foodborne disease acquired from food consumed away from home: A case study in Brazil. Food Control 2022, 133, 108602. [Google Scholar] [CrossRef]
- Langsrud, S.; Veflen, N.; Allison, R.; Crawford, B.; Izso, T.; Kasza, G.; Lecky, D.; Nicolau, A.I.; Scholderer, J.; Skuland, S.E.; et al. A trans disciplinary and multi actor approach to develop high impact food safety messages to consumers: Time for a revision of the WHO—Five keys to safer food? Trends Food Sci. Technol. 2023, 133, 87–98. [Google Scholar] [CrossRef]
- Schneider, G.; Schweitzer, B.; Steinbach, A.; Pertics, B.Z.; Cox, A.; Kőrösi, L. Antimicrobial Efficacy and Spectrum of Phosphorous-Fluorine Co-Doped TiO2 Nanoparticles on the Foodborne Pathogenic Bacteria Campylobacter jejuni, Salmonella Typhimurium, Enterohaemorrhagic E. coli, Yersinia enterocolitica, Shewanella putrefaciens, Listeria monocytogenes and Staphylococcus aureus. Foods 2021, 10, 1786. [Google Scholar] [CrossRef]
- Orsi, H.; Guimaraes, F.F.; Leite, D.S.; Guerra, S.T.; Joaquim, S.F.; Pantoja, J.C.F.; Hernandes, R.T.; Lucheis, S.B.; Ribeiro, M.G.; Langoni, H.; et al. Characterization of mammary pathogenic Escherichia coli reveals the diversity of Escherichia coli isolates associated with bovine clinical mastitis in Brazil. J. Dairy Sci. 2023, 106, 1403–1413. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Azlin-Hasim, S.; Cruz-Romero, M.; Cummins, E.; Kerry, J.P.; Morris, M.A. Antimicrobial effect of benzoic and sorbic acid salts and nano-solubilisates against Staphylococcus aureus, Pseudomonas fluorescens and chicken microbiota biofilms. Food Control 2020, 107, 106786. [Google Scholar] [CrossRef]
- D’Amore, T.; Di Taranto, A.; Vita, V.; Berardi, G.; Iammarino, M. Development and Validation of an Analytical Method for Nitrite and Nitrate Determination in Meat Products by Capillary Ion Chromatography (CIC). Food Anal. Methods 2019, 12, 1813–1822. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, W.; Zheng, Y.; Xiong, J.; Gao, C.; Hu, S. Occurrence, distribution, bioaccumulation, and ecological risk of bisphenol analogues, parabens and their metabolites in the Pearl River Estuary, South China. Ecotoxicol. Environ. Saf. 2019, 180, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, M.; Saleem, A.; Alsaiari, R.; Muhammad, N.; Latif, U.; Tariq, M.; Almohana, A.; Rahim, A. SiO(2)/Al(2)O(3)/C grafted 3-n propylpyridinium silsesquioxane chloride-based non-enzymatic electrochemical sensor for determination of carcinogenic nitrite in food products. Food Chem. 2022, 369, 130970. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Renwick, S.; Vancuren, S.J.; Robinson, A.V.; Gianetto-Hill, C.; Allen-Vercoe, E.; Barat, J.M. Impact of food preservatives based on immobilized phenolic compounds on an in vitro model of human gut microbiota. Food Chem. 2023, 403, 134363. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Q.; Dou, N.; Li, Y.; Ma, Y.; Liu, Y.; Wu, M.; Wei, X.; Miao, Y.; Chen, L.; et al. Evaporative concentration and high-pressure homogenization for improving the quality attributes and functionality of goat milk yogurt. LWT-Food Sci. Technol. 2023, 184, 115016. [Google Scholar] [CrossRef]
- Li, Y.-x.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef]
- Mohammadzadeh-Aghdash, H.; Sohrabi, Y.; Mohammadi, A.; Shanehbandi, D.; Dehghan, P.; Ezzati Nazhad Dolatabadi, J. Safety assessment of sodium acetate, sodium diacetate and potassium sorbate food additives. Food Chem. 2018, 257, 211–215. [Google Scholar] [CrossRef]
- Vasilaki, A.; Hatzikamari, M.; Stagkos-Georgiadis, A.; Goula, A.M.; Mourtzinos, I. A natural approach in food preservation: Propolis extract as sorbate alternative in non-carbonated beverage. Food Chem. 2019, 298, 125080. [Google Scholar] [CrossRef]
- Zarzecka, U.; Zadernowska, A.; Chajecka-Wierzchowska, W.; Adamski, P. High-pressure processing effect on conjugal antibiotic resistance genes transfer in vitro and in the food matrix among strains from starter cultures. Int. J. Food Microbiol. 2023, 388, 110104. [Google Scholar] [CrossRef]
- Liu, B.; Yu, K.; Ahmed, I.; Gin, K.; Xi, B.; Wei, Z.; He, Y.; Zhang, B. Key factors driving the fate of antibiotic resistance genes and controlling strategies during aerobic composting of animal manure: A review. Sci. Total Environ. 2021, 791, 148372. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Robertson, R.C.; Bwakura-Dangarembizi, M.; Prendergast, A.J.; Manges, A.R. Antibiotic use and resistance in children with severe acute malnutrition and human immunodeficiency virus infection. Int. J. Antimicrob. Agents 2023, 61, 106690. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jin, L.; Zhao, Y.; Jiang, L.; Yao, S.; Zhou, W.; Lin, K.; Cui, C. Annual trends and health risks of antibiotics and antibiotic resistance genes in a drinking water source in East China. Sci Total Environ. 2021, 791, 148152. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Korheina, D.K.A.; Fu, H.; Ge, X. Chronic exposure to dietary antibiotics affects intestinal health and antibiotic resistance gene abundance in oriental river prawn (Macrobrachium nipponense), and provokes human health risk. Sci Total Environ. 2020, 720, 137478. [Google Scholar] [CrossRef]
- Li, M.; Kong, J.; Chen, Y.; Li, Y.; Xuan, H.; Liu, M.; Zhang, Q.; Liu, J. Comparative interaction study of soy protein isolate and three flavonoids (Chrysin, Apigenin and Luteolin) and their potential as natural preservatives. Food Chem. 2023, 414, 135738. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, A.; Araya-Cloutier, C.; Vincken, J.P.; Abee, T.; den Besten, H.M.W. Impact of food-relevant conditions and food matrix on the efficacy of prenylated isoflavonoids glabridin and 6,8-diprenylgenistein as potential natural preservatives against Listeria monocytogenes. Int. J. Food Microbiol. 2023, 390, 110109. [Google Scholar] [CrossRef]
- Yu, W.; Guo, J.; Mao, L.; Wang, Q.; Liu, Y.; Xu, D.; Ma, J.; Luo, C. Glucose promotes cell growth and casein synthesis via ATF4/Nrf2-Sestrin2- AMPK-mTORC1 pathway in dairy cow mammary epithelial cells. Anim. Biotechnol. 2023, 1–11. [Google Scholar] [CrossRef]
- Kieronczyk, B.; Rawski, M.; Mikolajczak, Z.; Swiatkiewicz, S.; Jozefiak, D. Nisin as a Novel Feed Additive: The Effects on Gut Microbial Modulation and Activity, Histological Parameters, and Growth Performance of Broiler Chickens. Animial 2020, 10, 101. [Google Scholar] [CrossRef]
- Ma, J.; Li, T.; Wang, Q.; Xu, C.; Yu, W.; Yu, H.; Wang, W.; Feng, Z.; Chen, L.; Hou, J.; et al. Enhanced viability of probiotics encapsulated within synthetic/natural biopolymers by the addition of gum arabic via electrohydrodynamic processing. Food Chem. 2023, 413, 135680. [Google Scholar] [CrossRef]
- Ma, J.; Li, T.; Dou, N.; Li, Y.; Wang, Q.; Wu, M.; Miao, Y.; Li, J.; Su, C.; Chen, L.; et al. Consequences of vacuum evaporation on physicochemical properties, storage stability and in vitro digestion of fermented goat milk. Food Control 2023, 153, 109898. [Google Scholar] [CrossRef]
- Zafar, M.; Alam, S.; Sabir, M.; Saba, N.; Din, A.U.; Ahmad, R.; Khan, M.R.; Muhammad, A.; Dayisoylu, K.S. Isolation, characterization, bacteriocin production and biological potential of Bifidobacteria of ruminants. Anal. Biochem. 2022, 658, 114926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Bai, D.; Wei, Y.; Sun, J.; Luo, Y.; Zhao, J.; Liu, Y.; Wang, Q. Synergistic inhibition mechanism of pediocin PA-1 and L-lactic acid against Aeromonas hydrophila. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183346. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, N.; Zhu, Z.; Li, X.; Dai, D.; Pan, C.; Peng, D.; Sun, M. Three novel leaderless bacteriocins have antimicrobial activity against gram-positive bacteria to serve as promising food biopreservative. Microb. Cell Fact. 2022, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ma, H.; Sun, M.; Lin, Y.; Bai, F.; Li, J.; Zhang, B. A novel bacteriocin DY4-2 produced by Lactobacillus plantarum from cutlassfish and its application as bio-preservative for the control of Pseudomonas fluorescens in fresh turbot (Scophthalmus maximus) fillets. Food Control 2018, 89, 22–31. [Google Scholar] [CrossRef]
- Deng, S.; Liu, S.; Li, X.; Liu, H.; Li, F.; Liu, K.; Zeng, H.; Zeng, X.; Xin, B. Thuricins: Novel Leaderless Bacteriocins with Potent Antimicrobial Activity Against Gram-Positive Foodborne Pathogens. J. Agric. Food Chem. 2022, 70, 9990–9999. [Google Scholar] [CrossRef]
- Calvo, H.; Mendiara, I.; Arias, E.; Blanco, D.; Venturini, M.E. The role of iturin A from B. amyloliquefaciens BUZ-14 in the inhibition of the most common postharvest fruit rots. Food Microbiol. 2019, 82, 62–69. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Huang, Y.; Liu, X.; Dou, N.; Zhang, X.; Hou, J.; Ma, J. Physicochemical stability and in vitro digestibility of goat milk affected by freeze-thaw cycles. Food Chem. 2023, 404, 135680. [Google Scholar] [CrossRef]
- Mills, S.; Griffin, C.; O’Connor, P.M.; Serrano, L.M.; Meijer, W.C.; Hill, C.; Ross, R.P.; Björkroth, J. A Multibacteriocin Cheese Starter System, Comprising Nisin and Lacticin 3147 in Lactococcus lactis, in Combination with Plantaricin from Lactobacillus plantarum. Appl. Environ. Microbiol. 2017, 83, e00799-17. [Google Scholar] [CrossRef]
- Sheoran, P.; Tiwari, S.K. Synergistically-acting Enterocin LD3 and Plantaricin LD4 Against Gram-Positive and Gram-Negative Pathogenic Bacteria. Probiotics Antimicrob. Proteins 2020, 13, 542–554. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Q.; Wu, Y.; Chen, Z.; Liu, Y.; Fang, Z.; Deng, Q. Purification, characterization and structural identification of a novel bacteriocin produced by marine original Enterococcus durans YQ-6, and its inhibition of Listeria monocytogenes. LWT-Food Sci. Technol. 2023, 173, 114329. [Google Scholar] [CrossRef]
- Salazar-Marroquin, E.L.; Galan-Wong, L.J.; Moreno-Medina, V.R.; Reyes-Lopez, M.A.; Pereyra-Alferez, B. Bacteriocins synthesized by Bacillus thuringiensis: Generalities and potential applications. Rev. Med. Microbiol. 2016, 27, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Delshadi, R.; Jafari, S.M.; Williams, L. Nanoencapsulated nisin: An engineered natural antimicrobial system for the food industry. Trends Food Sci. Technol. 2019, 94, 20–31. [Google Scholar] [CrossRef]
- Wu, M.; Ma, Y.; Dou, X.; Zohaib Aslam, M.; Liu, Y.; Xia, X.; Yang, S.; Wang, X.; Qin, X.; Hirata, T.; et al. A review of potential antibacterial activities of nisin against Listeria monocytogenes: The combined use of nisin shows more advantages than single use. Food Res. Int. 2023, 164, 112363. [Google Scholar] [CrossRef] [PubMed]
- Forestier, A.; Belguesmia, Y.; Krier, F.; Drider, D.; Dhulster, P.; Firdaous, L. Recovery of nisin from culture supernatants of Lactococcus lactis by ultrafiltration: Flux properties and separation efficiency. Food Bioprod. Process. 2022, 136, 196–210. [Google Scholar] [CrossRef]
- Hassan, H.; St-Gelais, D.; Gomaa, A.; Fliss, I. Impact of Nisin and Nisin-Producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and Bacterial Ecosystem of Cheese Matrices. Foods 2021, 10, 898. [Google Scholar] [CrossRef]
- Nieva, S.G.; Jagus, R.J.; Agüero, M.V.; Fernandez, M.V. Fruit and vegetable smoothies preservation with natural antimicrobials for the assurance of safety and quality. LWT-Food Sci. Technol. 2022, 154, 112663. [Google Scholar] [CrossRef]
- Meral, R.; Alav, A.; Karakas, C.; Dertli, E.; Yilmaz, M.T.; Ceylan, Z. Effect of electrospun nisin and curcumin loaded nanomats on the microbial quality, hardness and sensory characteristics of rainbow trout fillet. LWT-Food Sci. Technol. 2019, 113, 108292. [Google Scholar] [CrossRef]
- Ma, J.; Xu, C.; Yu, H.; Feng, Z.; Yu, W.; Gu, L.; Liu, Z.; Chen, L.; Jiang, Z.; Hou, J. Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology. Food Hydrocoll. 2021, 111, 106381. [Google Scholar] [CrossRef]
- Liu, A.; Wan, Q.; Li, J.; Li, Q.; Hu, K.; Ao, X.; Chen, S.; He, L.; Hu, X.; Hu, B.; et al. Rose bud extract as a natural antimicrobial agent against Staphylococcus aureus: Mechanisms and application in maintaining pork safety. LWT-Food Sci. Technol. 2023, 176, 114527. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Ying, J.P.; Xin, W.G.; Yang, L.Y.; Li, X.Z.; Zhang, Q.L. Antibacterial activity and action target of phenyllactic acid against Staphylococcus aureus and its application in skim milk and cheese. J. Dairy Sci. 2022, 105, 9463–9475. [Google Scholar] [CrossRef]
- Wu, M.; Dong, Q.; Song, X.; Xu, L.; Xia, X.; Aslam, M.Z.; Ma, Y.; Qin, X.; Wang, X.; Liu, Y.; et al. Effective combination of nisin and sesamol against Listeria monocytogenes. LWT-Food Sci. Technol. 2023, 176, 114546. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- To, H.T.A.; Chhetri, V.; Settachaimongkon, S.; Prakitchaiwattana, C. Stress tolerance-Bacillus with a wide spectrum bacteriocin as an alternative approach for food bio-protective culture production. Food Control 2022, 133, 105898. [Google Scholar] [CrossRef]
- Thanjavur, N.; Sangubotla, R.; Lakshmi, B.A.; Rayi, R.; Mekala, C.D.; Reddy, A.S.; Viswanath, B. Evaluating the antimicrobial and apoptogenic properties of bacteriocin (nisin) produced by Lactococcus lactis. Process Biochem. 2022, 122, 76–86. [Google Scholar] [CrossRef]
- Yi, L.; Chen, S.; Li, G.; Ren, J.; Zhou, R.; Zeng, K. Prevalence of antibiotic resistance pathogens in online fresh-cut fruit from Chongqing, China and controlling Enterococcus faecalis by bacteriocin GF-15. LWT-Food Sci. Technol. 2022, 165, 113678. [Google Scholar] [CrossRef]
- Daba, G.M.; Mostafa, F.A.; Saleh, S.A.A.; Elkhateeb, W.A.; Awad, G.; Nomiyama, T.; Zendo, T.; El-Dein, A.N. Purification, amino acid sequence, and characterization of bacteriocin GA15, a novel class IIa bacteriocin secreted by Lactiplantibacillus plantarum GCNRC_GA15. Int. J. Biol. Macromol. 2022, 213, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, S.; Dong, S.; Zhang, Y.; Ismael, M.; Wang, S.; Shi, C.; Yang, J.; Wang, X.; Lu, X. Interaction of Companilactobacillus crustorum MN047-derived bacteriocins with gut microbiota. Food Chem. 2022, 396, 133730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, S.; Zhang, T.; Wu, Y.; Ma, J.; Zhao, L.; Li, X.; Zhang, J. Characterization and antibacterial modes of action of bacteriocins from Bacillus coagulans CGMCC 9951 against Listeria monocytogenes. LWT-Food Sci. Technol. 2022, 160, 113272. [Google Scholar] [CrossRef]
- Ceruso, M.; Liu, Y.; Gunther, N.W.; Pepe, T.; Anastasio, A.; Qi, P.X.; Tomasula, P.M.; Renye, J.A. Anti-listerial activity of thermophilin 110 and pediocin in fermented milk and whey. Food Control 2021, 125, 107941. [Google Scholar] [CrossRef]
- Yi, Y.; Li, P.; Zhao, F.; Zhang, T.; Shan, Y.; Wang, X.; Liu, B.; Chen, Y.; Zhao, X.; Lü, X. Current status and potentiality of class II bacteriocins from lactic acid bacteria: Structure, mode of action and applications in the food industry. Trends Food Sci. Technol. 2022, 120, 387–401. [Google Scholar] [CrossRef]
- Łepecka, A.; Szymański, P.; Okoń, A.; Zielińska, D. Antioxidant activity of environmental lactic acid bacteria strains isolated from organic raw fermented meat products. LWT-Food Sci. Technol. 2023, 174, 114440. [Google Scholar] [CrossRef]
- Field, D.; Ross, R.P.; Hill, C. Developing bacteriocins of lactic acid bacteria into next generation biopreservatives. Curr. Opin. Food Sci. 2018, 20, 1–6. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Hols, P.; Ledesma-Garcia, L.; Gabant, P.; Mignolet, J. Mobilization of Microbiota Commensals and Their Bacteriocins for Therapeutics. Trends Microbiol. 2019, 27, 690–702. [Google Scholar] [CrossRef] [PubMed]
- da Silva Sabo, S.; Vitolo, M.; Gonzalez, J.M.D.; Oliveira, R.P.S. Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Res. Int. 2014, 64, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, N.; Stuckey, D.C.; Ariff, A.B.; Faizal Wong, F.W. Novel approaches to purifying bacteriocin: A review. Crit Rev Food Sci. Nutr. 2018, 58, 2453–2465. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, L. Encapsulated natural antimicrobials: A promising way to reduce microbial growth in different food systems. Food Control 2021, 123, 107678. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Wu, J.; Zang, M.; Wang, S.; Zhao, B.; Bai, J.; Xu, C.; Shi, Y.; Qiao, X. Nisin: From a structural and meat preservation perspective. Food Microbiol. 2023, 111, 104207. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Belguesmia, Y.; Bendjeddou, K.; Kempf, I.; Boukherroub, R.; Drider, D. Heterologous Biosynthesis of Five New Class II Bacteriocins From Lactobacillus paracasei CNCM I-5369 With Antagonistic Activity Against Pathogenic Escherichia coli Strains. Front. Microbiol. 2020, 11, 1198. [Google Scholar] [CrossRef]
- Kyriakou, P.K.; Ekblad, B.; Kristiansen, P.E.; Kaznessis, Y.N. Interactions of a class IIb bacteriocin with a model lipid bilayer, investigated through molecular dynamics simulations. Biochim. Biophys. Acta 2016, 1858, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsen, C.; Brede, D.A.; Nes, I.F.; Diep, D.B. Circular bacteriocins: Biosynthesis and mode of action. Appl. Environ. Microbiol. 2014, 80, 6854–6862. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Saris, P.E.; Takala, T.M. Genetic characterization and expression of leucocin B, a class IId bacteriocin from Leuconostoc carnosum 4010. Res. Microbiol. 2015, 166, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhu, X.; Zhao, H.; Nie, T.; Lu, F.; Lu, Z.; Lu, Y. A class III bacteriocin with broad-spectrum antibacterial activity from Lactobacillus acidophilus NX2-6 and its preservation in milk and cheese. Food Control 2021, 121, 107597. [Google Scholar] [CrossRef]
- Eghbal, N.; Viton, C.; Gharsallaoui, A. Nano and microencapsulation of bacteriocins for food applications: A review. Food Biosci. 2022, 50, 102173. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Zhang, X.; Wu, H.; Zou, Y.; Li, P.; Sun, C.; Xu, W.; Liu, F.; Wang, D. Class III bacteriocin Helveticin-M causes sublethal damage on target cells through impairment of cell wall and membrane. J. Ind. Microbiol. Biotechnol. 2018, 45, 213–227. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; He, Y.; Zhang, Y.; She, Q.; Chai, Y.; Li, P.; Shang, Q. Characterization of Subtilin L-Q11, a Novel Class I Bacteriocin Synthesized by Bacillus subtilis L-Q11 Isolated From Orchard Soil. Front. Microbiol. 2019, 10, 484. [Google Scholar] [CrossRef]
- Halami, P.M. Sublichenin, a new subtilin-like lantibiotics of probiotic bacterium Bacillus licheniformis MCC 2512T with antibacterial activity. Microb. Pathog. 2019, 128, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, T.M.; Mendonca, C.M.N.; Vieira, V.B.; Robl, D.; de Melo Franco, B.D.G.; Todorov, S.D.; Tome, E.; O’Connor, P.M.; Converti, A.; Araujo, W.L.; et al. Pediocin PA-1 production by Pediococcus pentosaceus ET34 using non-detoxified hemicellulose hydrolysate obtained from hydrothermal pretreatment of sugarcane bagasse. Bioresour. Technol. 2021, 338, 125565. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Saravanakumar, K.; Daliri, E.B.; Kim, J.H.; Lee, J.K.; Jo, H.Y.; Kim, S.H.; Ramakrishnan, S.R.; Madar, I.H.; Wei, S.; et al. Unveiling the potentials of bacteriocin (Pediocin L50) from Pediococcus acidilactici with antagonist spectrum in a Caenorhabditis elegans model. Int. J Biol. Macromol. 2020, 143, 555–572. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, Y.; Li, B.; Ou, J.; Zhang, J.; Chen, Y.; Peng, X.; Chen, L. Molecular cloning and antimicrobial activity of enterolysin A and helveticin J of bacteriolysins from metagenome of Chinese traditional fermented foods. Food Control 2013, 31, 499–507. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Shan, C.; Xia, X.; Wang, Y.; Dong, M.; Zhou, J. Novel bacteriocin produced by Lactobacillus alimentarius FM-MM 4 from a traditional Chinese fermented meat Nanx Wudl: Purification, identification and antimicrobial characteristics. Food Control 2017, 77, 290–297. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Yang, L.-Y.; Ying, J.-P.; Fu, C.-M.; Wu, G.; Li, X.-R.; Zhang, Q.-L. A novel bacteriocin RSQ01 with antibacterial activity and its application and metabolomic mechanism in milk preservation. Food Control 2023, 151, 109823. [Google Scholar] [CrossRef]
- Ibarguren, C.; Guitian, M.V.; Lenz, R.M.; Cecilia, S.M.; Audisio, M.C. Response of sensitive and resistant Listeria monocytogenes strains against bacteriocins produced by different Enterococcus spp. strains. Int. J. Food Microbiol. 2022, 382, 109928. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, X.; Fan, P.; Qiao, B.; Xie, M.; Huang, T.; Xiong, T. Identification and characterization of a novel pH and heat stable bacteriocin-like substance lactococcin036019 with food preserving potential. Food Control 2023, 148, 109682. [Google Scholar] [CrossRef]
- Bauer, R.; Chikindas, M.L.; Dicks, L.M. Purification, partial amino acid sequence and mode of action of pediocin PD-1, a bacteriocin produced by Pediococcus damnosus NCFB 1832. Int. J. Food Microbiol. 2005, 101, 17–27. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Zhao, F.; Xiao, H.; Han, Y.; Zhou, Z. The mode of action of bacteriocin CHQS, a high antibacterial activity bacteriocin produced by Enterococcus faecalis TG2. Food Control 2019, 96, 470–478. [Google Scholar] [CrossRef]
- Rodriguez-Sojo, M.J.; Ruiz-Malagon, A.J.; Rodriguez-Cabezas, M.E.; Galvez, J.; Rodriguez-Nogales, A. Limosilactobacillus fermentum CECT5716: Mechanisms and Therapeutic Insights. Nutrients 2021, 13, 1016. [Google Scholar] [CrossRef] [PubMed]
- Ben Amara, C.; Kim, L.; Oulahal, N.; Degraeve, P.; Gharsallaoui, A. Using complexation for the microencapsulation of nisin in biopolymer matrices by spray-drying. Food Chem. 2017, 236, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Lounes-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.M.; El-Sayed, H.S. Antimicrobial nanoemulsion formulation based on thyme (Thymus vulgaris) essential oil for UF labneh preservation. J. Mater. Res. Technol. 2021, 10, 1029–1041. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Liu, G.; Nie, R.; Liu, Y.; Mehmood, A. Combined antimicrobial effect of bacteriocins with other hurdles of physicochemic and microbiome to prolong shelf life of food: A review. Sci Total Environ. 2022, 825, 154058. [Google Scholar] [CrossRef]
- Jannat, B.; Mirza Alizadeh, A.; Farshi, P.; Dadgarnejad, M.; Hosseini, H.; Hashempour-Baltork, F.; Jafari, S.M. Anti-biofilm activity of essential oils in fruit and vegetable: A systematic review. Food Control 2023, 152, 109875. [Google Scholar] [CrossRef]
- Gottardo, F.M.; Biduski, B.; Santos, L.F.d.; Santos, J.S.d.; Rodrigues, L.B.; Santos, L.R.d. Microencapsulated oregano and cinnamon essential oils as a natural alternative to reduce Listeria monocytogenes in Italian salami. Food Biosci. 2022, 50, 102146. [Google Scholar] [CrossRef]
- Fu, C.; Lan, X.; Yuan, J.; Li, C.; Li, L.; Yu, Z.; Tan, T.; Yuan, M.; Du, F. Research on the optimization, key chemical constituents and antibacterial activity of the essential oil extraction process of Thuja koraiensis Nakai. J. Microbiol. Methods 2022, 194, 106435. [Google Scholar] [CrossRef]
- Kang, Z.; Chen, S.; Zhou, Y.; Ullah, S.; Liang, H. Rational construction of citrus essential oil nanoemulsion with robust stability and high antimicrobial activity based on combination of emulsifiers. Innov. Food Sci. Emerg. Technol. 2022, 80, 103110. [Google Scholar] [CrossRef]
- Coimbra, A.; Carvalho, F.; Duarte, A.P.; Ferreira, S. Antimicrobial activity of Thymus zygis essential oil against Listeria monocytogenes and its application as food preservative. Innov. Food Sci. Emerg. Technol. 2022, 80, 103077. [Google Scholar] [CrossRef]
- Wang, H.; Guo, L.; Liu, L.; Han, B.; Niu, X. Composite chitosan films prepared using nisin and Perilla frutescense essential oil and their use to extend strawberry shelf life. Food Biosci. 2021, 41, 101037. [Google Scholar] [CrossRef]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The mechanisms of action of carvacrol and its synergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Li, Q.; Yu, S.; Han, J.; Wu, J.; You, L.; Shi, X.; Wang, S. Synergistic antibacterial activity and mechanism of action of nisin/carvacrol combination against Staphylococcus aureus and their application in the infecting pasteurized milk. Food Chem. 2022, 380, 132009. [Google Scholar] [CrossRef]
- Razavi Rohani, S.M.; Moradi, M.; Mehdizadeh, T.; Saei-Dehkordi, S.S.; Griffiths, M.W. The effect of nisin and garlic (Allium sativum L.) essential oil separately and in combination on the growth of Listeria monocytogenes. LWT Food Sci. Technol. 2011, 44, 2260–2265. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Hashemzadeh, M.S.; Nazarizadeh, A.; Neyriz-Naghadehi, M.; Tat, M.; Ghalavand, M.; Dorostkar, R. Chemical composition and antibacterial properties of Ocimum basilicum, Salvia officinalis and Trachyspermum ammi essential oils alone and in combination with nisin. Res. J. Pharmacogn. 2016, 3, 51–58. [Google Scholar]
- Canciu, A.; Cernat, A.; Tertis, M.; Botarca, S.; Bordea, M.A.; Wang, J.; Cristea, C. Proof of Concept for the Detection with Custom Printed Electrodes of Enterobactin as a Marker of Escherichia coli. Int. J. Mol. Sci. 2022, 23, 9884. [Google Scholar] [CrossRef]
- Ghrairi, T.; Hani, K. Enhanced bactericidal effect of enterocin A in combination with thyme essential oils against L. monocytogenes and E. coli O157:H7. J. Food Sci. Technol. 2015, 52, 2148–2156. [Google Scholar] [CrossRef]
- Papadochristopoulos, A.; Kerry, J.P.; Fegan, N.; Burgess, C.M.; Duffy, G. Natural Anti-Microbials for Enhanced Microbial Safety and Shelf-Life of Processed Packaged Meat. Foods 2021, 10, 1598. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Lekjing, S. A chitosan-based edible film with clove essential oil and nisin for improving the quality and shelf life of pork patties in cold storage. RSC Adv. 2020, 10, 17777–17786. [Google Scholar] [CrossRef] [PubMed]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Yang, X.; Liu, X.; Hong, H.; Luo, Y. Effects of oregano essential oil and nisin on the shelf life of modified atmosphere packed grass carp (Ctenopharyngodon idellus). LWT-Food Sci. Technol. 2021, 147, 111609. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control 2020, 107, 106771. [Google Scholar] [CrossRef]
- Khorsandi, A.; Eskandari, M.H.; Aminlari, M.; Shekarforoush, S.S.; Golmakani, M.T. Shelf-life extension of vacuum packed emulsion-type sausage using combination of natural antimicrobials. Food Control 2019, 104, 139–146. [Google Scholar] [CrossRef]
- Tao, R.; Sedman, J.; Ismail, A. Antimicrobial activity of various essential oils and their application in active packaging of frozen vegetable products. Food Chem. 2021, 360, 129956. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Kontogiorgos, V.; Daygon, V.D.; Fitzgerald, M.A. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica Oleracea L. Var. Capitata L.) Seedlings Grown under Controlled Conditions. Sustainability 2020, 12, 1871. [Google Scholar] [CrossRef]
- Fais, A.; Delogu, G.L.; Floris, S.; Era, B.; Medda, R.; Pintus, F. Euphorbia characias: Phytochemistry and Biological Activities. Plants 2021, 10, 1468. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Chen, H.; Li, H.; Zhou, Q.; Tong, P.; Liu, X. Antibacterial and antioxidant properties of clove extract applied in the production of dry-cured duck. Food Sci. Technol. 2023, 185, 115153. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Raal, A.; Laidmäe, I.; Topelius, N.S.; Komisarenko, M.; Komissarenko, A. Pharmaceutical 3D-printing of nanoemulsified eucalypt extracts and their antimicrobial activity. Eur. J. Pharm. Sci. 2023, 187, 106487. [Google Scholar] [CrossRef]
- Gado, D.A.; Abdalla, M.A.; Erhabor, J.O.; Ehlers, M.M.; McGaw, L.J. In vitro anti-biofilm effects of Loxostylis alata extracts and isolated 5-demethyl sinensetin on selected foodborne bacteria. S. Afr. J. Bot. 2023, 156, 29–34. [Google Scholar] [CrossRef]
- Saleh, E.; Morshdy, A.E.; El-Manakhly, E.; Al-Rashed, S.F.; Hetta, H.; Jeandet, P.; Yahia, R.; El-Saber Batiha, G.; Ali, E. Effects of Olive Leaf Extracts as Natural Preservative on Retailed Poultry Meat Quality. Foods 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, F.; Xie, X.; Xie, C.; Li, A.; Xia, N.; Gong, X.; Zhang, H. Development and characterization of chitosan/guar gum active packaging containing walnut green husk extract and its application on fresh-cut apple preservation. Int. J. Biol. Macromol. 2022, 209, 1307–1318. [Google Scholar] [CrossRef]
- He, B.; Chen, Y.; Yu, S.; Hao, Y.; Wang, F.; Qu, L. Food plant extracts for sleep-related skin health: Mechanisms and prospects. Food Biosci. 2022, 49, 101951. [Google Scholar] [CrossRef]
- Royani, A.; Hanafi, M.; Dewi, N.; Lotulung, P.; Eka Prastya, M.; Verma, C.; Manaf, A.; Alfantazi, A. Isolation and identification of bioactive compounds from Tinospora cordifolia stem extracts as antibacterial materials in seawater environments. Arab. J. Chem. 2023, 16, 105014. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Wu, J.; He, Y.; Yang, H. Elucidating antimicrobial mechanism of nisin and grape seed extract against Listeria monocytogenes in broth and on shrimp through NMR-based metabolomics approach. Int. J. Food Microbiol. 2020, 319, 108494. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Zhao, L.; He, Y.; Yang, H. Antimicrobial kinetics of nisin and grape seed extract against inoculated Listeria monocytogenes on cooked shrimps: Survival and residual effects. Food Control 2020, 115, 107278. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control 2020, 110, 107018. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innov. Food Sci. Emerg. Technol. 2016, 36, 287–293. [Google Scholar] [CrossRef]
- Bai, F.; Guo, D.; Wang, Y.; Zhang, S.; Li, J.; Zhi, K.; Shi, C.; Xia, X. The combined bactericidal effect of nisin and thymoquinone against Listeria monocytogenes in Tryptone Soy Broth and sterilized milk. Food Control 2022, 135, 108771. [Google Scholar] [CrossRef]
- Fernández, M.V.; Agüero, M.V.; Jagus, R.J. Green tea extract: A natural antimicrobial with great potential for controlling native microbiota, Listeria innocua and Escherichia coli in fresh-cut beet leaves. J. Food Saf. 2017, 38, e12374. [Google Scholar] [CrossRef]
- Djenane, D.; Aboudaou, M.; Djenane, F.; Garcia-Gonzalo, D.; Pagan, R. Improvement of the Shelf-Life Status of Modified Atmosphere Packaged Camel Meat Using Nisin and Olea europaea Subsp. laperrinei Leaf Extract. Foods 2020, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Bagatini, L.; Zandoná, G.P.; Hoffmann, J.F.; de Souza Cardoso, J.; Teixeira, F.C.; Moroni, L.S.; Junges, A.; Kempka, A.P.; Stefanello, F.M.; Rombaldi, C.V. Evaluation of Eugenia uniflora L. leaf extracts obtained by pressurized liquid extraction: Identification of chemical composition, antioxidant, antibacterial, and allelopathic activity. Sustain. Chem. Pharm. 2023, 35, 101214. [Google Scholar] [CrossRef]
- Yu, F.; Chen, C.; Chen, S.; Wang, K.; Huang, H.; Wu, Y.; He, P.; Tu, Y.; Li, B. Dynamic changes and mechanisms of organic acids during black tea manufacturing process. Food Control 2022, 132, 108535. [Google Scholar] [CrossRef]
- Shetty, R.; Petersen, F.R.; Podduturi, R.; Molina, G.E.S.; Wätjen, A.P.; Madsen, S.K.; Zioga, E.; Øzmerih, S.; Hobley, T.J.; Heiner Bang-Berthelsen, C. Fermentation of brewer’s spent grain liquids to increase shelf life and give an organic acid enhanced ingredient. LWT-Food Sci. Technol. 2023, 182, 114911. [Google Scholar] [CrossRef]
- Hu, J.; Wu, J.; Wang, M.; Jiao, W.; Chen, Q.; Du, Y.; Chen, X.; Yang, X.; Fu, M. p-coumaric acid prevents Colletotrichum gloeosporioides by inhibiting membrane targeting and organic acid metabolism. Postharvest Biol. Technol. 2023, 204, 112447. [Google Scholar] [CrossRef]
- Long, S.F.; Xu, Y.T.; Pan, L.; Wang, Q.Q.; Wang, C.L.; Wu, J.Y.; Wu, Y.Y.; Han, Y.M.; Yun, C.H.; Piao, X.S. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Vongkamjan, K. Combined effects of Salmonella phage cocktail and organic acid for controlling Salmonella Enteritidis in chicken meat. Food Control 2022, 133, 108653. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Yang, H. Integrated metabolomics of “big six” Escherichia coli on pea sprouts to organic acid treatments. Food Res. Int. 2022, 157, 111354. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; He, Y.; Wang, Y.; Yang, H. NMR-based metabolomic investigation on antimicrobial mechanism of Salmonella on cucumber slices treated with organic acids. Food Control 2022, 137, 108973. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Toselli, M.; Sabia, C.; Messi, P.; de Niederhausern, S.; Bondi, M.; Iseppi, R. Effectiveness of polymeric coated films containing bacteriocin-producer living bacteria for Listeria monocytogenes control under simulated cold chain break. Food Microbiol. 2018, 76, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ajingi, Y.U.S.; Ruengvisesh, S.; Khunrae, P.; Rattanarojpong, T.; Jongruja, N. The combined effect of formic acid and Nisin on potato spoilage. Biocatal. Agric. Biotechnol. 2020, 24, 101523. [Google Scholar] [CrossRef]
- Ajingi, Y.u.S.; Rodpan, S.; Usman, J.N.; Koga, Y.; Jongruja, N. Synergistic effect of Nisin with acetic and propionic acids inactivates Bacillus subtilis on meat and potato. Biocatal. Agric. Biotechnol. 2022, 41, 102317. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Jeong, D.-Y.; Lee, S.-B.; Kim, S.-R. Control of Listeria monocytogenes and Escherichia coli O157:H7 in enoki mushrooms (Flammulina velutipes) by combined treatments with organic acids, nisin, and ultrasound. Food Control 2021, 129, 108204. [Google Scholar] [CrossRef]
- Zhou, J.; Velliou, E.; Hong, S.H. Investigating the effects of nisin and free fatty acid combined treatment on Listeria monocytogenes inactivation. LWT-Food Sci. Technol. 2020, 133, 110115. [Google Scholar] [CrossRef]
- Zhao, X.; Zhen, Z.; Wang, X.; Guo, N. Synergy of a combination of nisin and citric acid against Staphylococcus aureus and Listeria monocytogenes. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess 2017, 34, 2058–2068. [Google Scholar] [CrossRef]
- Zhao, G.; Kempen, P.J.; Zheng, T.; Jakobsen, T.H.; Zhao, S.; Gu, L.; Solem, C.; Ruhdal Jensen, P. Synergistic bactericidal effect of nisin and phytic acid against Escherichia coli O157:H7. Food Control 2023, 144, 109324. [Google Scholar] [CrossRef]
- Zhang, P.; Gänzle, M.; Yang, X.; Elkins, C.A. Complementary Antibacterial Effects of Bacteriocins and Organic Acids as Revealed by Comparative Analysis of Carnobacterium spp. from Meat. Appl. Environ. Microbiol. 2019, 85, e01227-19. [Google Scholar] [CrossRef]
- Ning, Y.; Hou, L.; Ma, M.; Li, M.; Zhao, Z.; Zhang, D.; Wang, Z.; Jia, Y. Synergistic antibacterial mechanism of sucrose laurate combined with nisin against Staphylococcus aureus and its application in milk beverage. LWT-Food Sci. Technol. 2022, 158, 113145. [Google Scholar] [CrossRef]
- Kirazli, S.; Tunca, S. NISIN and gilaburu (Viburnum opulus L.) combination is a cost-effective way to control foodborne Staphylococcus aureus. Food Control 2022, 142, 109213. [Google Scholar] [CrossRef]
- Prudêncio, C.V.; Mantovani, H.C.; Cecon, P.R.; Prieto, M.; Vanetti, M.C.D. Temperature and pH influence the susceptibility of Salmonella Typhimurium to nisin combined with EDTA. Food Control 2016, 61, 248–253. [Google Scholar] [CrossRef]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem 2022, 369, 130956. [Google Scholar] [CrossRef]
- Field, D.; Baghou, I.; Rea, M.C.; Gardiner, G.E.; Ross, R.P.; Hill, C. Nisin in Combination with Cinnamaldehyde and EDTA to Control Growth of Escherichia coli Strains of Swine Origin. Antibiotics 2017, 6, 35. [Google Scholar] [CrossRef]
- Castellano, P.; Belfiore, C.; Vignolo, G. Combination of bioprotective cultures with EDTA to reduce Escherichia coli O157:H7 in frozen ground-beef patties. Food Control 2011, 22, 1461–1465. [Google Scholar] [CrossRef]
- Chang, S.H.; Chen, Y.J.; Tseng, H.J.; Hsiao, H.I.; Chai, H.J.; Shang, K.C.; Pan, C.L.; Tsai, G.J. Applications of Nisin and EDTA in Food Packaging for Improving Fabricated Chitosan-Polylactate Plastic Film Performance and Fish Fillet Preservation. Membranes 2021, 11, 852. [Google Scholar] [CrossRef]
- Liu, J.; Meng, F.; Du, Y.; Nelson, E.; Zhao, G.; Zhu, H.; Caiyin, Q.; Zhang, Z.; Qiao, J. Co-production of Nisin and γ-Aminobutyric Acid by Engineered Lactococcus lactis for Potential Application in Food Preservation. Front. Microbiol. 2020, 11, 49. [Google Scholar] [CrossRef]
- Liu, H.; Pei, H.; Han, Z.; Feng, G.; Li, D. The antimicrobial effects and synergistic antibacterial mechanism of the combination of ε-Polylysine and nisin against Bacillus subtilis. Food Control 2015, 47, 444–450. [Google Scholar] [CrossRef]
- Alessandrini, R.; Brown, M.K.; Pombo-Rodrigues, S.; Bhageerutty, S.; He, F.J.; MacGregor, G.A. Nutritional Quality of Plant-Based Meat Products Available in the UK: A Cross-Sectional Survey. Nutrients 2021, 13, 4225. [Google Scholar] [CrossRef]
- Lopes Lessa, V.; Harumi Omura, M.; Pacheco, S.; Basilio de Oliveira, E.; Ribeiro de Barros, F.A. Obtention and evaluation of physico-chemical and techno-functional properties of macauba (Acrocomia aculeata) kernel protein isolate. Food Res. Int. 2022, 161, 111848. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Han, L.; Li, Z.; Gu, M.; Xiao, Z.; Lu, F. Combination of Chitosan, Tea Polyphenols, and Nisin on the Bacterial Inhibition and Quality Maintenance of Plant-Based Meat. Foods 2022, 11, 1524. [Google Scholar] [CrossRef] [PubMed]


| Classify | Characteristic | Source | Represent | References |
|---|---|---|---|---|
| Class I | A modified peptide containing 19–50 amino acids. | L. lactis Bacillus | Nisin Subtilin L-Q 11 | [80,81] |
| Class II | Unmodified membrane active peptide with molecular weight less than 10 kDa. | Pediococcus acidilactici | Pediocin PA-1 Pediocin L50 | [82,83] |
| Class III | Thermal instability, high molecular weight > 30 kDa | Lactobacillus | Helveticin J | [84] |
| Class IV | A complex protein composed of one or more chemical groups. | Lactobacillus | Lactocin 27 | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Guo, J.; Liu, Y.; Xue, X.; Wang, X.; Wei, L.; Ma, J. Potential Impact of Combined Inhibition by Bacteriocins and Chemical Substances of Foodborne Pathogenic and Spoilage Bacteria: A Review. Foods 2023, 12, 3128. https://doi.org/10.3390/foods12163128
Yu W, Guo J, Liu Y, Xue X, Wang X, Wei L, Ma J. Potential Impact of Combined Inhibition by Bacteriocins and Chemical Substances of Foodborne Pathogenic and Spoilage Bacteria: A Review. Foods. 2023; 12(16):3128. https://doi.org/10.3390/foods12163128
Chicago/Turabian StyleYu, Wei, Jinqi Guo, Yuanyuan Liu, Xiaoge Xue, Xiangru Wang, Lili Wei, and Jiage Ma. 2023. "Potential Impact of Combined Inhibition by Bacteriocins and Chemical Substances of Foodborne Pathogenic and Spoilage Bacteria: A Review" Foods 12, no. 16: 3128. https://doi.org/10.3390/foods12163128
APA StyleYu, W., Guo, J., Liu, Y., Xue, X., Wang, X., Wei, L., & Ma, J. (2023). Potential Impact of Combined Inhibition by Bacteriocins and Chemical Substances of Foodborne Pathogenic and Spoilage Bacteria: A Review. Foods, 12(16), 3128. https://doi.org/10.3390/foods12163128