Broccoli, Amaranth, and Red Beet Microgreen Juices: The Influence of Cold-Pressing on the Phytochemical Composition and the Antioxidant and Sensory Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microgreen Sample
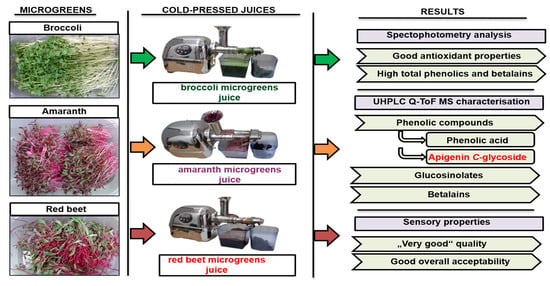
2.2. Preparation of Cold-Pressed Microgreen Juices
2.3. Preparation of Microgreens for Chromatographic Analysis
2.4. Preparation of Cold-Pressed Microgreen Juices for Chromatographic Analysis
2.5. UHPLC Q-ToF MS of Microgreens and Cold-Pressed Microgreen Juices
2.6. Proximate Compositions of Cold-Pressed Microgreen Juices
2.7. Total Phenolics, Flavonoids, Betalains, and Chlorophyll Content in Microgreen Juices
2.8. Antioxidant Properties of Cold-Pressed Microgreen Juices
2.9. Sensory Properties of Cold-Pressed Microgreen Juices
2.9.1. Overall Quality Evaluation
2.9.2. Consumer Acceptance Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. UHPLC Q-ToF MS Profile of Bioactive Compounds of Broccoli, Amaranth, and Red Beet Microgreens
3.2. Proximate Composition of Microgreen Juices
3.3. UHPLC Q-ToF MS Profile of Microgreen Juices
3.4. Total Phenolic, Flavonoid, Betalain, and Chlorophyll Content in Cold-Pressed Microgreen Juices
3.5. Antioxidant Properties of the Cold-Pressed Microgreen Juices
3.6. Sensory Properties of Cold-Pressed Microgreen Juices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebert, A.W. Sprouts and Microgreens-Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Shree, B.; Sharma, D.; Kumar, S.; Kumar, V.; Sharma, R.; Saini, R. Vegetable microgreens: The gleam of next generation super foods, their genetic enhancement, health benefits and processing approaches. Food Res. Int. 2022, 155, 111038. [Google Scholar] [CrossRef]
- Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Antiproliferative Effect of Bioaccessible Fractions of Four Brassicaceae Microgreens on Human Colon Cancer Cells Linked to Their Phytochemical Composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef]
- Renna, M.; Di Gioia, F.; Leoni, B.; Mininni, C.; Santamaria, P. Culinary Assessment of Self-Produced Microgreens as Basic Ingredients in Sweet and Savory Dishes. J. Culin. Sci. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef]
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae microgreens: A novel and promissory source of sustainable bioactive compounds. Curr. Res. Food Sci. 2023, 6, 100480. [Google Scholar] [CrossRef] [PubMed]
- Castellaneta, A.; Losito, I.; Cisternino, G.; Leoni, B.; Santamaria, P.; Calvano, C.D.; Bianco, G.; Cataldi, T.R.I. All Ion Fragmentation Analysis Enhances the Untargeted Profiling of Glucosinolates in Brassica Microgreens by Liquid Chromatography and High-Resolution Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2022, 33, 2108–2119. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Luo, Y.; Wang, T.T.Y.; Chen, P. Profiling of Polyphenols and Glucosinolates in Kale and Broccoli Microgreens Grown under Chamber and Windowsill Conditions by Ultrahigh-Performance Liquid Chromatography High-Resolution Mass Spectrometry. ACS Food Sci. Technol. 2022, 2, 101–113. [Google Scholar] [CrossRef]
- Demir, K.; Sarıkamış, G.; Çakırer Seyrek, G. Effect of LED lights on the growth, nutritional quality and glucosinolate content of broccoli, cabbage and radish microgreens. Food Chem. 2023, 401, 134088. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Tomas, M.; Zhang, L.; Zengin, G.; Lucini, L.; Capanoglu, E. Red beet (Beta vulgaris) and amaranth (Amaranthus sp.) microgreens: Effect of storage and in vitro gastrointestinal digestion on the untargeted metabolomic profile. Food Chem. 2020, 332, 127415. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-Specific Modulatory Effects of Select Spectral Bandwidths on the Nutritive and Phytochemical Composition of Microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In Vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, S.; Wang, Y.; Liu, J.; Wang, J.; Lu, Y. Broccoli microgreens juice reduces body weight by enhancing insulin sensitivity and modulating gut microbiota in high-fat diet-induced C57BL/6J obese mice. Eur. J. Nutr. 2021, 60, 3829–3839. [Google Scholar] [CrossRef] [PubMed]
- Senthilnathan, K.; Muthusamy, S. Process optimization & kinetic modeling study for fresh microgreen (Alternanthera sessilis) juice treated under thermosonication. Prep. Biochem. Biotechnol. 2022, 52, 433–442. [Google Scholar]
- Ali, N.; Popović, V.; Koutchma, T.; Warriner, K.; Zhu, Y. Effect of thermal, high hydrostatic pressure, and ultraviolet-C processing on the microbial inactivation, vitamins, chlorophyll, antioxidants, enzyme activity, and color of wheatgrass juice. J. Food Process. Eng. 2019, 43, e13036. [Google Scholar] [CrossRef]
- Skoczylas, Ł.; Korus, A.; Tabaszewska, M.; Gędoś, K.; Szczepańska, E. Evaluation of the quality of fresh and frozen wheatgrass juices depending on the time of grass harvest: SKOCZYLAS et al. J. Food Process. Preserv. 2017, 42, e13401. [Google Scholar] [CrossRef]
- Klopsch, R.; Baldermann, S.; Voss, A.; Rohn, S.; Schreiner, M.; Neugart, S. Bread Enriched With Legume Microgreens and Leaves—Ontogenetic and Baking-Driven Changes in the Profile of Secondary Plant Metabolites. Front. Chem. 2018, 6, 322. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A.; Rasane, P.; Dey, A.; Choudhury, A.; Singh, J.; Kaur, S.; Dhawan, K.; Kaur, D. Optimization of a process for microgreen and fruit-based functional beverage. An. Acad. Bras Cienc. 2020, 92, e20190596. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Hussain, A.; Goksen, G.; Ali, M.; Khalil, A.A.; Zeng, X.-A.; Jambrak, A.R.; Lorenzo, J.M. Probing the impact of sustainable emerging sonication and DBD plasma technologies on the quality of wheat sprouts juice. Ultrason. Sonochem. 2023, 92, 106257. [Google Scholar] [CrossRef]
- Ferruzza, S.; Natella, F.; Ranaldi, G.; Murgia, C.; Rossi, C.; Trošt, K.; Mattivi, F.; Nardini, M.; Maldini, M.; Giusti, A.M.; et al. Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation. Pharmaceuticals 2016, 9, 48. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Ziros, P.G.; Chen, J.G.; Groopman, J.D.; Kensler, T.W.; Sykiotis, G.P. Broccoli sprout beverage is safe for thyroid hormonal and autoimmune status: Results of a 12-week randomized trial. Food Chem. Toxicol. 2019, 126, 1–6. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Michell, K.; Isweiri, H.; Newman, S.; Bunning, M.; Bellows, L.; Dinges, M.; Grabos, L.; Rao, S.; Foster, M.; Heuberger, A.; et al. Microgreens: Consumer sensory perception and acceptance of an emerging functional food crop. J. Food Sci. 2020, 85, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lamadrid, M.; Martínez Zamora, L.; Castillejo, N.; Cattaneo, C.; Pagliarini, E.; Artés-Hernández, F. How does the phytochemical composition of sprouts and microgreens from Brassica vegetables affect the sensory profile and consumer acceptability? Postharvest Biol. Technol. 2023, 203, 112411. [Google Scholar] [CrossRef]
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; De Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043. [Google Scholar] [CrossRef]
- Chen, H.; Tong, X.; Tan, L.; Kong, L. Consumers’ acceptability and perceptions toward the consumption of hydroponically and soil grown broccoli microgreens. J. Agric. Food Res. 2020, 2, 100051. [Google Scholar] [CrossRef]
- Kolarević, T.; Milinčić, D.D.; Vujović, T.; Gašić, U.M.; Prokić, L.; Kostić, A.Ž.; Cerović, R.; Stanojevic, S.P.; Tešić, Ž.L.; Pešić, M.B. Phenolic compounds and antioxidant properties of field-grown and in vitro leaves, and calluses in blackberry and blueberry. Horticulturae 2021, 7, 420. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Acharya, J.; Gautam, S.; Neupane, P.; Niroula, A. Pigments, ascorbic acid, and total polyphenols content and antioxidant capacities of beet (Beta vulgaris) microgreens during growth. Int. J. Food Prop. 2021, 24, 1175–1186. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Špirović Trifunović, B.; Nedić, N.; Gašić, U.M.; Tešić, Ž.L.; Stanojević, S.P.; Pešić, M.B. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Tian, Z.; Ma, Y.; Yang, Z.; Ma, Z.; Wang, X.; Li, Y.; Jiang, H. Rapid screening and characterization of glucosinolates in 25 Brassicaceae tissues by UHPLC-Q-exactive orbitrap-MS. Food Chem. 2021, 365, 130493. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods Anal. Methods 2010, 2, 310. [Google Scholar] [CrossRef]
- Cataldi, T.R.; Rubino, A.; Lelario, F.; Bufo, S.A. Naturally occurring glucosinolates in plant extracts of rocket salad (Eruca sativa L.) identified by liquid chromatography coupled with negative ion electrospray ionization and quadrupole ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sun, Y.; Tang, Q.; Zhang, H.; Cheng, Z. Isolation, identification, biological estimation, and profiling of glucosinolates in Isatis indigotica roots. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 645–656. [Google Scholar] [CrossRef]
- Isayenkova, J.; Wray, V.; Nimtz, M.; Strack, D.; Vogt, T. Cloning and functional characterisation of two regioselective flavonoid glucosyltransferases from Beta vulgaris. Phytochemistry 2006, 67, 1598–1612. [Google Scholar] [CrossRef]
- da Silva, L.G.S.; Morelli, A.P.; Pavan, I.C.B.; Tavares, M.R.; Pestana, N.F.; Rostagno, M.A.; Simabuco, F.M.; Bezerra, R.M.N. Protective effects of beet (Beta vulgaris) leaves extract against oxidative stress in endothelial cells in vitro. Phytother. Res. 2020, 34, 1385–1396. [Google Scholar] [CrossRef]
- Xie, G.-R.; Chen, H.-J. Comprehensive Betalain Profiling of Djulis (Chenopodium formosanum) Cultivars Using HPLC-Q-Orbitrap High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 15699–15715. [Google Scholar] [CrossRef] [PubMed]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Identification of heat-induced degradation products from purified betanin, phyllocactin and hylocerenin by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2603–2616. [Google Scholar] [CrossRef]
- Sawicki, T.; Martinez-Villaluenga, C.; Frias, J.; Wiczkowski, W.; Peñas, E.; Bączek, N.; Zieliński, H. The effect of processing and in vitro digestion on the betalain profile and ACE inhibition activity of red beetroot products. J. Funct. Foods 2019, 55, 229–237. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Stintzing, F.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis: General Guidance for Design of Test Rooms. ISO: Genèva, Switzerland, 2007. Available online: https://www.iso.org/standard/76667.html (accessed on 3 January 2024).
- ISO 11136:2014; Sensory Analysis—Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area. ISO: Genèva, Switzerland, 2014. Available online: https://www.iso.org/standard/50125.html (accessed on 3 January 2024).
- Senate of the University of Belgrade. The Code of Professional Ethics of the University of Belgrade. Off. Gaz. Repub. Serb. 2016, 189, 16. [Google Scholar]
- Tomic, N.; Djekic, I.; Hofland, G.; Smigic, N.; Udovicki, B.; Rajkovic, A. Comparison of Supercritical CO2-Drying, Freeze-Drying and Frying on Sensory Properties of Beetroot. Foods 2020, 9, 1201. [Google Scholar] [CrossRef]
- ISO 8586:2023; Sensory Analysis—Selection and Training of Sensory Assessors. ISO: Genèva, Switzerland, 2023. Available online: https://www.iso.org/standard/36385.html (accessed on 3 January 2024).
- Petrović, M.; Veljović, S.; Tomić, N.; Zlatanović, S.; Tosti, T.; Vukosavljević, P.; Gorjanović, S. Formulation of Novel Liqueurs from Juice Industry Waste: Consumer Acceptance, Phenolic Profile and Preliminary Monitoring of Antioxidant Activity and Colour Changes during Storage. Food Technol. Biotechnol. 2021, 59, 282–294. [Google Scholar] [CrossRef]
- Tomic, N.; Dojnov, B.; Miocinovic, J.; Tomasevic, I.; Smigic, N.; Djekic, I.; Vujcic, Z. Enrichment of yoghurt with insoluble dietary fiber from triticale–A sensory perspective. LWT 2017, 80, 59–66. [Google Scholar] [CrossRef]
- Zeng, W.; Yang, J.; Yan, G.; Zhu, Z. CaSO(4) Increases Yield and Alters the Nutritional Contents in Broccoli (Brassica oleracea L. Var. italica) Microgreens under NaCl Stress. Foods 2022, 11, 3485. [Google Scholar]
- Di Bella, M.C.; Toscano, S.; Arena, D.; Moreno, D.A.; Romano, D.; Branca, F. Effects of Growing Cycle and Genotype on the Morphometric Properties and Glucosinolates Amount and Profile of Sprouts, Microgreens and Baby Leaves of Broccoli (Brassica oleracea L. var. italica Plenck) and Kale (B. oleracea L. var. acephala DC.). Agronomy 2021, 11, 1685. [Google Scholar] [CrossRef]
- Bello, C.; Maldini, M.; Baima, S.; Scaccini, C.; Natella, F. Glucoraphanin and sulforaphane evolution during juice preparation from broccoli sprouts. Food Chem. 2018, 268, 249–256. [Google Scholar] [CrossRef]
- Lorizola, I.; Furlan, C.P.; Portovedo, M.; Milanski, M.; Botelho, P.; Bezerra, R.; Sumere, B.; Rostagno, M.; Capitani, C. Beet Stalks and Leaves (Beta vulgaris L.) Protect Against High-Fat Diet-Induced Oxidative Damage in the Liver in Mice. Nutrients 2018, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Antonini, E.; Frati, A.; Scarpa, E.S. C-Glycosyl Flavonoids from Beta vulgaris Cicla and Betalains from Beta vulgaris rubra: Antioxidant, Anticancer and Antiinflammatory Activities-A Review. Phytother. Res. 2017, 31, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Celli, G.B.; Brooks, M.S.-L. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins—A current review. Food Res. Int. 2017, 100, 501–509. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]




| RT | Formula | Calculated Mass | mDa | Compound Name | m/z Exact Mass | Major MS Fragments (Base Peak) | BC | BC1 | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1.72 | C11H18NO9S2− | 372.0423 | −5.00 | Gluconapin | 372.0473 | 130(100), 195, 259, 275, 241, 291, 139 | - | + | [9,37,38] |
| 6.71 | C15H20NO9S2− | 422.0579 | −2.60 | Gluconasturtiin | 422.0605 | 205(100), 247, 164, 259, 275, 180, 226, 244, 342 | + | - | |
| 6.14 | C16H19N2O9S2− | 447.0532 | −4.15 | Glucobrassicin | 447.0574 | 130(100), 259, 205, 447, 275, 165, 195 | + | + | |
| 1.77 | C16H19N2O10S2− | 463.0481 | −4.35 | 4-Hydroxy- glucobrassicin | 463.0525 | 169(100), 160, 221, 259, 275, 195, 463, 205, 285, 383, 267, 241, 186, 176 | + | + | |
| 2.56 | C16H19N2O10S2− | 463.0481 | −4.35 | 5-Hydroxy- glucobrassicin | 463.0525 | 169(100), 160, 221, 259, 267, 275, 195, 205, 285, 463 | + | + | |
| 7.13 | C17H21N2O10S2− | 477.0638 | −4.54 | Neo- glucobrassicin | 477.0683 | 477(100), 259, 275, 284, 235, 195, 241, 145 | + | + | |
| 7.88 | C17H21N2O10S2− | 477.0638 | −4.54 | 4-Methoxy- glucobrassicin | 477.0683 | 167(100), 259, 205, 241, 275, 282, 285, 195, 315, 447 | + | + |
| No | Compounds Name | RT | Formula | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (% of Base Peaks) | Samples (mg/100 g) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AM | BC | RB | ||||||||
| Phenolic acid and derivatives | ||||||||||
| 1 | Dihydroxy- benzoic acid hexoside isomer I b | 3.83 | C13H15O9− | 315.07216 | 315.07504 | −2.88 | 108.0228 (100), 109.0298 (41), 110.0330 (4), 152.0132 (58), 153.0199 (19), 154.0198 (2) | 10.08 ± 0.08 B | 104.14 ± 1.14 A | / |
| 2 | Vanillic acid hexoside isomer I b | 4.45 | C14H17O9− | 329.08781 | 329.08943 | −1.63 | 108.021 (100), 109.0263 (8), 113.0218 (4), 123.0449 (35), 124.0473 (3), 125.0240 (8), 152.0109 (76), 153.0136 (10), 167.0364 (34), 169.019 (3) | 4.23 ± 0.03 B | 11.25 ± 0.06 A | / |
| 3 | Hydroxy- benzoic acid dihexoside b | 5.18 | C24H19O10− | 467.09837 | 467.10054 | −2.17 | 137.0246 (100), 138.0278 (9), 299.0771 (2), 431.1188 (5) | 25.08 ± 0.10 | / | / |
| 4 | Vanillic acid hexoside isomer II b | 5.18 | C14H17O9− | 329.08781 | 329.08927 | −1.46 | 108.0229 (100), 109.0265 (8), 122.0367 (3), 123.0464 (39), 124.0504 (4), 152.0129 (61), 153.0172 (4), 167.0369 (32), 168.0409 (4) | 7.34 ± 0.04 B | 38.53 ± 0.54 A | 4.41 ± 0.12 C |
| 5 | Hydroxy- benzoic acid pentosyl hexoside isomer I b | 5.52 | C18H23O12− | 431.11950 | 431.12157 | −2.07 | 137.0246 (100), 138.0287 (8) | 95.15 ± 0.37 A | / | 2.58 ± 0.08 B |
| 6 | Carboxy- vanillic acid b | 5.59 | C9H7O6− | 211.02430 | 211.02751 | −3.21 | 108.0235 (39), 109.0275 (2), 121.0287 (2), 122.0386 (100), 123.0453 (35), 124.0465 (3) | / | 28.99 ± 0.22 | / |
| 7 | Dihydroxy- benzoic acid hexoside isomer II b | 5.65 | C13H15O9− | 315.07216 | 315.07845 | −6.29 | 108.0222 (8), 109.0305 (100), 110.0335 (14), 152.0114 (12), 153.0208 (61) | 10.33 ± 0.12 B | 19.23 ± 0.20 A | 3.83 ± 0.03 C |
| 8 | Vanillic acid pentosyl hexoside b | 5.79 | C19H25O13− | 461.13007 | 461.13684 | −6.77 | 108.0226 (5), 123.0448 (8), 152.0113 (18), 153.0166 (2), 167.0356 (100), 168.0383 (10) | 123.40 ± 0.83 | / | / |
| 9 | Sinapoyl syringic acid b | 5.92 | C20H19O9− | 403.10290 | 403.10103 | 1.87 | 138.0306 (51), 153.0543 (31), 154.0590 (12), 161.0362 (27), 182.0204 (34), 189.0316 (89), 190.0316 (8), 197.0445 (100), 198.0494 (13), 203.0441 (7), 204.0558 (15) | / | 7.93 ± 0.05 | / |
| 10 | Dihydroxy- benzoic acid pentosyl hexoside b | 6.05 | C18H23O13− | 447.11442 | 447.11850 | −4.08 | 101.0249 (4), 108.022 (14), 109.0298 (15), 151.0397 (3), 152.0118 (100), 153.0169 (13), 154.0189 (1), 161.0464 (3), 315.0738 (2), 447.1157 (37) | 65.56 ± 0.98 A | / | 4.55 ± 0.15 B |
| 11 | Dihydroxy- benzoic acid pentoside b | 6.05 | C12H13O8− | 285.06159 | 285.06344 | −1.85 | 108.0221 (100), 109.0282 (19), 152.0118 (43), 153.0171 (9) | 2.65 ± 0.06 B | / | 59.79 ± 1.89 A |
| 12 | Syringic acid hexoside b | 6.32 | C15H19O10− | 359.09837 | 359.10153 | −3.15 | 101.0242 (59), 113.0246 (64), 121.0289 (38), 137.0261 (45), 138.0326 (71), 152.0488 (52), 153.0559 (56), 166.0279 (18), 181.0140 (41), 182.0234 (56), 196.0406 (22), 197.0449 (87), 211.0609 (20), 239.0567 (34), 359.1005 (100) | / | 54.42 ± 0.98 A | 17.16 ± 0.26 B |
| 13 | Coumaric acid pentoside b | 6.32 | C14H15O7− | 295.08180 | 295.08967 | −7.87 | 108.0206 (22), 127.1141 (13), 149.0220 (24), 151.1095 (19), 152.0415 (13), 163.0405 (100) | / | 15.80 ± 0.24 | / |
| 14 | Hydroxy- benzoic acid hexoside b | 6.45 | C13H15O8− | 299.07724 | 299.08261 | −5.37 | 108.0823 (8), 121.024 (11), 122.0373 (61), 123.0458 (51), 137.0252 (100), 138.027 (10) | 9.44 ± 0.04 C | 10.02 ± 0.13 B | 25.76 ± 0.54 A |
| 15 | Dihydroxy- benzoic acid dipentoside b | 6.66 | C17H21O12− | 417.10385 | 417.10765 | −3.80 | 108.022 (18), 109.0298 (23), 110.0326 (1), 151.0402 (4), 152.0121 (100), 153.0168 (13), 285.0628 (2), 417.1050 (25) | / | / | 252.35 ± 2.32 |
| 16 | Caffeic acid hexoside b | 6.66 | C15H17O9− | 341.08730 | 341.09286 | −5.56 | 134.0356 (5), 135.0466 (100), 136.0467 (9), 137.0575 (11), 145.0860 (5), 161.0255 (6), 164.0495 (6), 178.0266 (3), 179.0352 (61) | / | 16.47 ± 0.08 | / |
| 17 | Feruloyl quinic acid b | 6.86 | C17H19O9− | 367.10290 | 367.10878 | −5.88 | 111.0471 (4), 117.0343 (15), 120.9974 (9), 134.0387 (100), 135.0427 (14), 146.0620 (11), 149.0617 (8), 155.0341 (5), 173.0472 (5), 190.0531 (6), 191.0589 (6), 193.0521 (49), 194.0527 (6) | / | 21.67 ± 0.24 | / |
| 18 | Sinapic acid dihexoside b | 6.80 | C23H31O15− | 547.16630 | 547.17128 | −4.98 | 101.0242 (22), 113.0239 (10), 119.0346 (12), 149.0263 (9), 164.0477 (26), 179.0676 (13), 190.0262 (18), 205.0523 (80), 206.0563 (27), 221.0789 (40), 223.0616 (69), 247.0621 (100) | / | 35.71 ± 0.48 | / |
| 19 | Ferulic acid hexoside b | 7.07 | C16H19O9− | 355.10346 | 355.10802 | −4.56 | 111.009 (100), 112.0128 (7), 132.0233 (6), 134.0375 (85), 135.0408 (8), 149.0616 (20), 154.9994 (12), 160.0170 (73), 161.0204 (8), 175.0402 (59), 176.0450 (8), 178.0277 (44), 179.0308 (5), 193.0514 (28), 194.0534 (4) | 39.70 ± 0.14 A | 7.80 ± 0.13 B | / |
| 20 | Sinapic acid hexoside b,*** | 7.14 | C17H21O10− | 385.11402 | 385.11743 | −3.41 | 101.0262(2), 113.0255(2), 119.0222(2), 147.0115(2), 149.0268(4), 164.0499 (10), 175.0056 (12), 190.0296 (100), 191.0334 (13), 192.03511(2), 205.05315 (77), 206.0571 (13), 207.0594 (2), 223.0633 (3) | / | 214.46 ± 2.22 | / |
| 21 | Feruloyl isocitric acid b | 8.14 | C16H15O10− | 367.06707 | 367.07166 | −4.59 | 111.0092 (100), 112.0128 (7), 129.0199 (3), 134.0379 (2), 154.9992 (12), 173.0099 (3) | 175.78 ± 1.10 A | / | 6.59 ± 0.09 B |
| 22 | Sinapoyl malic acid b,*** | 8.35 | C15H15O9− | 339.07216 | 339.07690 | −4.74 | 115.0054 (34), 116.0085 (2), 121.0311 (8), 132.0237 (2), 133.0161 (29), 134.0199 (2), 147.0469 (7), 149.0263 (100), 150.0297 (10), 164.0499 (79), 165.0532 (9), 193.0168 (2), 208.0406 (3), 223.064 (6) | / | 159.12 ± 1.90 | / |
| 23 | Sinapic acid a | 8.35 | C11H11O5− | 223.06120 | 223.06408 | −2.89 | 104.0281 (4), 117.0361 (3), 121.0309 (100), 122.0342 (10), 132.0250 (1), 135.0460 (3), 149.0257 (62), 150.0288 (6), 163.0413 (2), 165.0227 (4), 193.0166 (9) | / | 526.06 ± 2.29 | / |
| 24 | Benzoyl malic acid b | 8.48 | C11H9O6− | 237.03990 | 237.04732 | −7.42 | 103.4469 (3), 115.0112 (3), 121.0295 (100), 122.0361 (8) | 53.27 ± 1.27 A | 4.45 ± 0.12 B | / |
| 25 | Disinapoyl-dihexoside b,*** | 8.75 | C34H41O19− | 753.22420 | 753.23231 | −8.11 | 119.0359 (3), 164.0496 (4), 179.0659 (3), 190.0294 (7), 205.0529 (100), 206.0565 (14), 208.0398 (3), 223.0637 (66), 224.0672 (8), 247.0642 (9), 265.0760 (4), 289.0751 (3), 529.1625 (42), 530.1661 (14), 531.1661 (3) | / | 122.51 ± 1.95 | / |
| 26 | Trisanapoyl-dihexoside b,*** | 9.29 | C45H51O23− | 959.28210 | 959.28907 | −6.97 | 205.0525 (75), 206.0564 (7), 223.0637 (31), 247.0641 (14), 265.0763 (7), 289.0759 (7), 511.1509 (28), 512.1537 (8), 529.1607 (30), 530.1613 (9), 735.2217 (100), 736.2243 (46), 737.2255 (13), 959.2905 (14) | / | 130.53 ± 1.96 | / |
| ∑ | 622.02 | 1529.08 | 377.03 | |||||||
| Apigenin C-glycosides **** | ||||||||||
| 27 | 2″-Hexosyl vitexin c | 7.82 | C27H31O15+ | 595.16630 | 595.17351 | −7.21 | 271.0608 (32), 283.0597 (17), 295.06144 (13), 313.0709 (100), 314.0746 (25), 337.0715 (20), 367.0819 (10), 379.0809 (9), 397.0928 (28), 398.0949 (9), 415.1031 (48), 416.1061 (14), 433.1138 (87), 434.1180 (26), 435.1191 (7) | / | / | 65.09 ± 0.31 |
| 28 | 2″-Pentosyl vitexin c | 7.95 | C26H29O14+ | 565.15570 | 565.16177 | −6.07 | 283.0599 (16), 295.0603 (6), 313.0714 (100), 314.0753 (24), 337.0711 (16), 343.0821 (7), 367.0818 (14), 379.0818 (11), 397.0923 (42), 398.097 (12), 415.1031 (68), 416.1074 (19), 433.1138 (90), 434.1172 (28), 565.1550 (14) | / | / | 87.04 ± 1.02 |
| 29 | 2″-Hexosyl-6″-malonyl vitexin c | 8.22 | C30H33O18+ | 681.16670 | 681.17274 | −6.04 | 271.0606 (19), 283.0604 (7), 295.0610 (21), 313.0712 (100), 314.0739 (24), 337.0706 (13), 345.1099 (7), 439.1031 (9), 457.1122 (8), 475.1238 (13), 483.0918 (7), 501.1043 (22), 502.1063 (8), 519.1149 (60), 520.1182 (23) | / | / | 67.35 ± 2.12 |
| 30 | 2″-Hexosyl-6″-acetyl vitexin c,* | 8.27 | C29H31O16− | 635.16120 | 635.16454 | −3.34 | 101.0257 (5), 175.0376 (6), 193.0516 (8), 293.0464 (100), 311.0613 (15), 337.0938 (7), 413.0880(21), 431.0900(5), 455.0981 (88), 473.1113 (12), 575.1456 (9) | / | / | 6.97 ± 0.06 |
| 31 | 2″-Pentosyl-6″-malonyl vitexin c | 8.36 | C29H31O17+ | 651.15610 | 651.15914 | −3.04 | 283.0600 (9), 295.0611 (19), 313.0713 (100), 314.0744 (23), 337.0707 (13), 379.0820 (8), 439.1029 (11), 445.1139 (8), 457.1149 (10), 475.1246 (7), 483.0936 (7), 501.1035 (20), 519.1146 (41), 520.1178 (15), 651.1564 (11) | / | / | 76.12 ± 0.79 |
| 32 | 2″-Hexosyl cytisoside c | 8.49 | C28H33O15+ | 609.18190 | 609.18544 | −3.54 | 285.0764 (34), 297.0764 (15), 309.0762 (14), 327.0874 (100), 351.0868 (18), 381.0977 (9), 393.0975 (8), 411.1082 (28), 429.1191 (47), 447.1296 (88) | / | / | 170.18 ± 1.63 |
| 33 | 2”-Pentosyl cytisoside c | 8.69 | C27H31O14+ | 579.17140 | 579.17423 | −2.83 | 297.0767 (15), 327.0872 (100), 328.0908 (25), 351.0863 (15), 357.0970 (9), 381.0975 (14), 393.0975 (10), 411.1088 (41), 412.1115 (13), 429.1193 (70), 430.1223 (23), 447.1296 (94), 448.1334 (31), 579.1716 (18), 580.1764 (7) | / | / | 184.04 ± 1.83 |
| 34 | 2”-Hexosyl-6”-malonyl cytisoside c,** | 8.83 | C31H35O18+ | 695.18230 | 695.18826 | −5.96 | 285.0763 (24), 297.0759 (9), 309.0767 (20), 327.0869 (100), 328.0904 (25), 351.0871 (12), 393.0976 (8), 453.1185 (10), 471.1286 (10), 489.1415 (15), 497.1094 (7), 515.1202 (24), 516.1235 (9), 533.1299 (76), 534.1330 (28) | / | / | 64.77 ± 1.35 |
| 35 | 2″-Hexosyl-6″-acetyl cytisoside c | 8.96 | C30H35O16+ | 651.19250 | 651.19507 | −2.57 | 297.0764 (6), 327.0871 (7), 369.0976 (22), 370.1010 (7), 381.0966 (4), 393.0958 (4), 411.1094 (12), 423.1086 (4), 429.1180 (10), 430.1229 (4), 471.1295 (14), 472.1314 (5), 489.1406 (100), 490.1438 (36), 491.1459 (8) | / | / | 194.21 ± 1.98 |
| 36 | 2″-Pentosyl-6″-acetyl cytisoside c,* | 9.08 | C29H31O15− | 619.16684 | 619.17055 | −3.70 | 101.0255 (18), 113.0246 (26), 131.0344 (12), 283.0643 (12), 307.0619 (100), 308.0676 (17), 325.0731 (62), 326.0740 (15), 337.0726 (44), 349.0726 (22), 367.0843 (33), 409.0936 (17), 427.1057 (16), 469.1162 (13), 619.1704 (16) | / | / | 35.15 ± 0.93 |
| ∑ | / | / | 950.92 | |||||||
| Other detected flavonoids | ||||||||||
| 37 | Kaempferol-3-O-(6″-hexosyl)hexoside-7-O-hexoside with HCOOH c,*** | 6.53 | C34H41O23− | 817.20390 | 817.20689 | −2.99 | 284.0375 (3), 285.0391 (4), 288.6129 (2), 299.0519 (2), 446.0897 (4), 447.0986 (48), 448.1022 (16), 489.0970 (3), 609.1533 (100), 610.1576 (39), 611.1627 (9), 612.1502 (2), 771.2062 (5), 772.2040 (3) | / | 6.50 ± 0.08 | / |
| 38 | Kaempferol-3-O-sinapoyl-trihexoside-7-O-hexoside c,*** | 7.04 | C50H59O30− | 1139.30910 | 1139.32320 | −14.10 | 1139.318 (100), 815.2109 (52), 977.2654 (43), 609.1536 (9), 284.0357 (8) | / | 26.27 ± 0.25 | / |
| 39 | Kaempferol-3-O-sinapoyl-dihexoside-7-O-hexoside c,*** | 7.07 | C44H51O25+ | 979.27190 | 979.27923 | −7.33 | 127.0423 (4), 207.0669 (95), 208.0701 (16), 225.0765 (4), 287.0564 (55), 288.0608 (11), 291.0869 (3), 351.1114 (31), 352.1174 (7), 369.1216 (100), 370.1249 (26), 371.1262 (6), 449.1127 (49) | / | 24.84 ± 0.72 | / |
| 40 | Quercetin 3-O-(6″-rhamnosyl)-hexoside c | 8.02 | C27H29O16− | 609.14611 | 609.14934 | −3.24 | 151.0039 (3), 178.9985 (4), 255.0319 (3), 271.0253 (7), 300.0283 (100), 301.0355 (73) | 24.37 ± 0.36 A | 2.01 ± 0.02 C | 3.06 ± 0.07 B |
| ∑ | 24.37 | 59.63 | 3.06 | |||||||
| ∑∑ | 646.38 | 1588.71 | 1331.0 | |||||||
| No | Compounds Name | RT | Formula | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (% of Base Peaks) | Samples (%) | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| AM | RB | |||||||||
| Betalains | ||||||||||
| 41 | (Iso)Amaranthin | 5.05 | C30H35N2O19+ | 727.18285 | 727.18431 | −1.45 | 150.0552(1), 389.0982 (100), 390.1014 (28), 391.1044 (5), 551.1509 (5), 552.1541 (2), 727.1837 (21) | 73.56 | 0.17 | [41,43,44] |
| 42 | (Iso)betanin | 5.72 | C24H27N2O13+ | 551.15130 | 551.15259 | −1.29 | 150.0549 (2), 343.0931 (2), 389.0987 (100), 390.1021 (29), 391.1041 (5), 551.1522 (4) | 5.22 | 49.20 | |
| 43 | 17-Decarboxy- (iso)amaranthin | 5.85 | C29H35N2O17+ | 683.19302 | 683.19430 | −1.27 | 150.056 (1), 345.1084(100), 346.1116 (27), 347.1135 (4), 507.1618 (9), 508.1649 (4), 683.1930 (31) | 17.35 | - | |
| 44 | (2, 15 or 17)- Decarboxy- (iso)betanin | 5.93 | C23H27N2O11+ | 507.16150 | 507.16233 | −0.83 | 106.0660 (2), 150.0549 (2), 299.1035 (1), 301.1186 (1), 345.1089 (100), 346.1124 (25), 347.1145 (4), 507.1617 (5) | 1.66 | 23.29 | |
| 45 | (2, 15 or 17)- Decarboxy(iso) betanin | 6.40 | C23H27N2O11+ | 507.16150 | 507.16406 | −2.56 | 150.0548 (2), 299.1030 (2), 301.1176 (1), 345.1088 (100), 346.1126 (25), 347.1144 (4), 507.1622 (2) | 2.22 | 23.55 | |
| 46 | (2, 15 or 17)- Decarboxy-(iso) betanidin | 7.01 | C17H17N2O6+ | 345.10811 | 345.11267 | −4.56 | 100.0392 (27), 106.0643 (37), 132.0449 (53), 144.0302 (47), 150.0541 (99), 151.0626 (62), 152.0708 (36), 202.0881 (34), 209.0726 (36), 227.0862 (35), 253.0849 (61), 255.1138 (65), 281.0767 (49), 299.1034 (43), 345.1061 (100) | - | 3.79 | |
| Total (%) | 100 | 100 | ||||||||
| Microgreen Juices | Family and Species | Yield of Juices (%) | Percentage of Dry Weight (%) | Percentage of Moisture (%) | pH Values | °Brix |
|---|---|---|---|---|---|---|
| BCJ | Brassica oleracea var. italica | 70.20 ± 0.15 a | 1.84 ± 0.05 b | 98.26 ± 0.05 b | 5.96 ± 0.01 c | 2.00 ± 0.01 a |
| RBJ | Beta vulgaris | 62.00 ± 0.10 b | 2.00 ± 0.01 a | 98.00 ± 0.01 c | 6.44 ± 0.01 b | 2.00 ± 0.01 a |
| AMJ | Amaranthus tricolour L. | 53.40 ± 0.15 c | 1.64 ± 0.01 c | 98.46 ± 0.01 a | 6.52 ± 0.01 a | 1.80 ± 0.01 b |
| No | Compounds Name | RT | Formula | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (% of Base Peaks) | Samples (mg/100 mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AMJ | BCJ | RBJ | ||||||||
| Phenolic acid and derivatives | ||||||||||
| 1a | Hydroxy- benzoic acid hexoside isomer I b | 1.68 | C13H15O8− | 299.07724 | 299.08054 | −3.30 | 137.0248 (100), 138.0301 (10) | / | 3.47 ± 0.03 | / |
| 2a | Shikimic quinic acid hexoside b | 2.15 | C22H23O15− | 527.10370 | 527.11099 | −7.29 | 143.0015 (3), 167.0342 (4), 173.0272 (2), 191.0555 (100), 192.0593 (10), 193.0580 (3), 353.0837 (4) | 0.92 ± 0.01 | / | / |
| 3a | Dihydroxy- benzoic acid isomer I b | 2.35 | C7H5O4− | 153.01933 | 153.02154 | −2.21 | 108.0229 (100), 109.0294 (81), 110.0311 (6) | / | 0.72 ± 0.02 | / |
| 4a | Hydroxy- benzoic acid dihexoside b | 3.46 | C24H19O10− | 467.09837 | 467.09935 | −0.98 | 137.0255 (100), 138.0284 (9), 299.0774 (2) | 2.40 ± 0.02 | / | / |
| 5a | Hydroxy- benzoic acid b | 3.91 | C7H5O3− | 137.02390 | 137.02614 | −2.24 | / | 14.97 ± 0.19 A | 5.15 ± 0.01 C | 9.81 ± 0.02 B |
| 6a | Dihydroxy- benzoic acid isomer III b | 4.23 | C7H5O4− | 153.01933 | 153.02021 | −0.88 | 106.9976 (65), 107.0293 (52), 107.053(20), 108.0218 (100), 122.9839 (14), 123.0203 (34), 135.0194 (11), 135.0538 (12) | / | 0.99 ± 0.03 | / |
| 7a | Vanillic acid pentosyl hexoside b | 4.43 | C19H25O13− | 461.13007 | 461.13222 | −2.16 | 108.0226 (5), 123.0461(7), 152.0122 (18), 153.0161 (3), 167.0374 (100), 168.0382 (10) | 6.86 ± 0.03 | / | / |
| 8a | Dihydroxy- benzoic acid pentoside b | 4.71 | C12H13O8− | 285.06159 | 285.06303 | −1.44 | 108.0231 (100), 109.0291 (22), 110.0312 (2), 152.0117 (47), 153.0164 (9), 154.0176 (2) | / | / | 7.33 ± 0.03 |
| 9a | Dihydroxy- benzoic acid pentosyl hexoside b | 5.32 | C18H23O13− | 447.11390 | 447.11985 | −5.95 | 101.02230(3), 108.0229 (13), 109.0289 (14), 136.0394 (11), 151.0374 (3), 152.0114 (100), 153.0161 (14), 161.0453 (3), 163.0387 (6), 315.0666 (3), 447.1152 (46) | 13.83 ± 0.07 | / | / |
| 10a | Benzoic acid derivative (like as carboxy benzoic acid) b | 5.66 | C8H5O4− | 165.01880 | 165.02126 | −2.46 | 105.0153(52), 105.0395 (58), 108.0156 (13), 120.0197(38), 121.0306 (100), 122.0288 (11), 123.9880 (27), 124.0190(39), 135.0394 (17), 147.8908 (8), 151.9801(24), 152.0114 (32) | / | 48.02 ± 0.02 | / |
| 11a | Hydroxy- benzoic acid hexoside isomer II b | 5.78 | C13H15O8− | 299.07724 | 299.08174 | −4.50 | 137.0252 (100), 138.0307 (9) | / | / | 1.06 ± 0.04 |
| 12a | Coumaroyl- quinic acid isomer I b | 5.78 | C16H17O8− | 337.09289 | 337.09853 | −5.64 | 111.0452 (5), 119.0519 (100), 120.0542 (11), 163.0406 (50), 164.0437 (7), 173.0448 (4), 191.0564 (60) | / | 55.84 ± 0.14 | / |
| 13a | Benzoic acid b | 5.80 | C7H5O2− | 121.02900 | 121.03002 | −1.02 | / | 3.68 ± 0.02 B | 14.80 ± 0.02 A | 0.46 ± 0.01 C |
| 14a | 5-O-Caffeoyl- quinic acid isomer I b | 6.19 | C16H17O9− | 353.08781 | 353.08964 | −1.83 | 135.0452 (1), 161.0242 (2), 173.0454 (1), 191.0554 (100) | / | 1.61 ± 0.02 | / |
| 15a | Coumaric acid hexoside b | 6.27 | C15H17O8− | 325.09230 | 325.09815 | −5.85 | 117.0354 (6), 119.0513 (100), 120.0544 (11), 163.0398 (24), 164.0436 (3) | 2.63 ± 0.03 | / | / |
| 16a | Carboxy hydroxybenzoic acid b | 6.53 | C8H5O5− | 181.01370 | 181.01425 | −0.55 | 107.0304(15), 107.0612 (12), 117.0185 (9), 119.0235 (100), 120.0294 (16), 134.0376(30), 135.0487 (14), 137.0287 (26) | / | 0.66 ± 0.01 | / |
| 17a | Sinapic acid hexoside b | 6.59 | C17H21O10− | 385.11402 | 385.11652 | −2.50 | 149.0249(21), 164.0481 (56), 165.0516 (8), 175.0042 (12), 179.0701(14), 190.0274 (100), 191.0325 (25), 205.0510 (99), 206.0569(45), 207.0492 (9), 217.0156 (11), 221.0806(14), 223.0620 (12) | / | 18.15 ± 0.02 | / |
| 18a | Ferulic acid hexoside b | 6.67 | C16H19O9− | 355.10346 | 355.10148 | 1.98 | 111.0102(50), 112.0133 (16), 113.0147 (18), 134.0379 (100), 135.0424(12), 149.0610 (28), 150.0672 (3), 154.9760 (5), 155.0063 (6), 157.0035 (3), 178.0270 (62), 179.0308 (9), 193.0504 (37), 194.0542 (6) | 1.41 ± 0.01 | / | / |
| 19a | Hydroxy- benzoyl malic acid b | 6.84 | C11H9O7− | 253.03480 | 253.03892 | −4.12 | 102.9829 (2), 103.0087 (2), 114.0580 (2), 121.0305(100), 122.0332(10), 123.0058 (2), 123.0383(2), 130.0424 (2) | 2.02 ± 0.02 | / | / |
| 20a | Coumaroyl- quinic acid isomer II b | 6.88 | C16H17O8− | 337.09289 | 337.09441 | −1.52 | 109.0311(2), 111.0446 (18), 112.0467 (2), 119.0508 (44), 120.0531 (5), 137.0257 (11), 138.0320 (1), 155.0348 (6), 163.0402 (26), 164.0441(4), 173.0455 (100), 174.0484(10), 191.0549 (3) | / | 1.16 ± 0.02 | / |
| 21a | Sinapic acid a | 7.88 | C11H11O5− | 223.06120 | 223.06222 | −1.03 | 105.0352(1), 121.0308 (100), 122.0339 (9), 134.0359 (1), 135.0456 (13), 136.0548 (1), 148.0172 (5), 149.0248 (50), 150.0277 (5), 163.0396 (13), 164.0469 (5), 165.0197 (27), 166.0219 (3), 193.0142(60), 194.0177 (7) | / | 60.00 ± 0.54 | / |
| 22a | Sinapoyl malic acid b | 7.94 | C15H15O9− | 339.07216 | 339.07540 | −3.24 | 115.0047 (47), 116.0085 (2), 117.0301 (1), 121.0313 (8), 132.0226 (2), 133.0156 (43), 134.0193 (2), 147.0462 (7), 149.0248 (100), 150.0291 (11), 164.0480 (86), 165.0519 (10), 179.0716 (2), 208.0385 (2), 223.0620 (7) | / | 134.18 ± 0.04 | / |
| 23a | Benzoylmalic acid b | 7.94 | C11H9O6− | 237.03990 | 237.04514 | −5.24 | 114.9839 (2), 115.0099 (3), 121.0310 (100), 122.0333 (10) | / | 2.09 ± 0.02 | / |
| 24a | Dihydroxy- benzoic acid dihexoside b | 8.15− | C21H19O13− | 479.08260 | 479.08873 | −6.13 | 108.0228(20), 109.0346 (21), 137.0257 (61), 152.0122 (67), 153.0151(18), 435.0914 (100) | / | 8.21 ± 0.08 | / |
| 25a | Hydroxyferulic acid b | 11.25− | C10H9O5− | 209.04500 | 209.04494 | 0.06 | 105.0353 (100), 107.0146(58), 121.0291 (23), 123.0439 (44), 125.0253 (13), 131.0141 (16), 149.02305 (77), 150.0276(13), 151.0024 (70), 165.0555 (18), 167.0333 (19), 191.0347(10), 193.0143 (63), 209.0157 (12) | / | 2.53 ± 0.04 | / |
| ∑ | 48.74 | 357.59 | 18.66 | |||||||
| Apigenin C-glycosides | ||||||||||
| 26a | 2″-Hexosyl vitexin c | 7.54 | C27H31O15+ | 595.16630 | 595.17146 | −5.16 | 271.0586(29), 283.0586 (17), 295.0580 (13), 313.0710 (100), 337.0691(18), 367.0794 (10), 379.0794 (8), 397.0905(29), 415.1016 (45), 433.1133 (88) | / | / | 28.81 ± 0.05 |
| 27a | 2″-Pentosyl vitexin c | 7.61 | C26H29O14+ | 565.15570 | 565.16052 | −4.82 | 283.0596(16), 313.0724 (100), 337.0699 (17), 343.0802 (9), 367.0806 (16), 379.0806 (11), 397.0921 (41), 415.1041(74), 433.1144 (100) | / | / | 34.45 ± 0.05 |
| 28a | 2″-Hexosyl-6″- acetyl vitexin c | 7.81 | C29H33O16+ | 637.17690 | 637.18029 | −3.39 | 283.0596(4), 295.0573 (4), 313.0710 (10), 337.0694 (3), 355.0789 (28), 367.0793 (3), 397.0898 (6), 415.1022 (10), 457.1121 (12), 475.1226 (100) | / | / | 4.34 ± 0.04 |
| 29a | 2″-Hexosyl-6″- malonyl vitexin c | 7.95 | C30H33O18+ | 681.16670 | 681.17004 | −3.34 | 271.0583(18), 283.0590 (8), 295.0587 (21), 313.0712 (100), 337.0684 (13), 379.0797 (7), 439.1005 (9), 457.1118 (9), 475.1212(14), 483.0908 (8), 501.1012 (23), 519.1140 (60) | / | / | 26.43 ± 0.05 |
| 30a | 2″-Hexosyl cytisoside c | 8.29 | C28H33O15+ | 609.18190 | 609.18759 | −5.69 | 285.0753(32), 297.0745 (16), 309.0745 (13), 327.0844 (100), 351.0852 (18), 381.0953 (8), 393.0952 (8), 411.1073 (28), 429.1186(50), 447.1272 (91) | / | / | 48.94 ± 0.12 |
| 31a | 2″-Rhamnosyl cytisoside c | 8.35 | C28H33O14+ | 593.18700 | 593.19311 | −6.11 | 297.0738(12), 327.0850 (57), 351.0832 (12), 357.0946 (7), 381.0956 (11), 393.0956 (8), 411.1058 (34), 429.1168 (58), 447.1273 (100) | / | / | 5.48 ± 0.06 |
| 32a | 2″-Pentosyl cytisoside c | 8.42 | C27H31O14+ | 579.17140 | 579.17499 | −3.59 | 297.0746(14), 327.0847 (100), 351.0851 (15), 357.0956 (9), 381.0957 (14), 393.0954 (9), 411.1078 (43), 429.1174(71), 447.1276 (95) | / | / | 46.00 ± 0.23 |
| 33a | 2″-Hexosyl-6″- malonyl cytisoside c | 8.56 | C31H35O18+ | 695.18230 | 695.19050 | −8.20 | 285.0741(24), 297.0736 (9), 309.0747(21), 327.0859 (100), 351.0840 (12), 393.0956 (8), 453.1166 (10), 471.1282 (12), 489.1373 (17), 497.1060 (8), 515.1173 (27), 533.1284 (77) | / | / | 17.90 ± 0.07 |
| 34a | Cytisoside (3′-Methyl vitexin) c | 8.62 | C22H23O10+ | 447.12910 | 447.13521 | −6.11 | 135.0459 (8), 297.0737 (51), 309.0717 (8), 327.0846 (100), 337.1007 (14), 351.0832(22), 357.0948 (14), 365.1001 (10), 381.0924(11), 393.0937 (15), 411.1024 (31), 429.1197 (16) | / | / | 4.71 ± 0.05 |
| 35a | 2″-Hexosyl-6″- acetyl cytisoside c | 8.69 | C30H35O16+ | 651.19250 | 651.19531 | −2.81 | 297.0739(3), 309.0738 (3), 327.0852 (8), 351.0839 (2), 369.0966 (27), 393.095(2), 411.1064 (7), 429.1165 (9), 471.1271 (10), 489.1382 (100) | / | / | 67.44 ± 0.34 |
| 36a | 2″-Hexuronyl-6″-acetyl cytisoside c | 8.76 | C30H33O17+ | 665.17180 | 665.17943 | −7.63 | 297.0742(10), 309.0736 (18), 327.0865 (100), 351.0839 (12), 453.1161(11), 459.1265 (11), 471.1254(12), 489.1366(10), 515.1169 (21), 533.1280 (49) | / | / | 18.49 ± 0.08 |
| 37a | 6″-Acetyl cytisoside c | 9.30 | C24H25O11+ | 489.13914 | 489.14587 | −6.73 | 297.0740(33), 309.074 (21), 327.0846 (95), 351.0846 (13), 369.0954 (100), 381.0946(18), 393.0946 (15), 411.1052 (34), 429.1160(45), 471.1267 (16) | / | / | 9.39 ± 0.04 |
| 38a | 2″-Malonyl-6″- acetyl- cytisoside c | 9.57 | C27H27O14+ | 575.14010 | 575.14610 | −6.00 | 127.0370(7), 129.1006 (7), 297.0701 (12), 309.0736(51), 327.0849 (66), 351.0842 (19), 369.0949 (100), 375.0937 (8), 393.0966 (13), 453.1132(9), 471.1253 (9) | / | / | 1.40 ± 0.01 |
| 39a | Apigenin a | 10.44 | C15H9O5− | 269.04500 | 269.05007 | −5.07 | 136.9884(53), 139.0059 (53), 141.0708 (26), 143.0506(19), 167.0342 (31), 169.0656 (44), 171.0446(35), 179.0495 (19), 195.0448 (50), 197.0606(25), 223.0392 (52), 241.0492 (43), 251.0359(19), 269.0453 (100) | 1.06 ± 0.02 B | / | 9.39 ± 0.05 A |
| ∑ | 1.06 | / | 323.17 | |||||||
| Other flavonoids | ||||||||||
| 40a | Kaempferol-3-O-sinapoyl- dihexoside-7-O-hexoside c | 6.85 | C44H49O25− | 977.25630 | 977.26510 | −8.80 | 815.2079 (100), 816.2111(54), 977.2594 (20), 609.1468 (13), 284.0332 (9), 446.085 (3) | / | 4.77 ± 0.07 | / |
| 41a | Chalcan-flavan 3-ol dimer c | 7.68 | C27H31O14− | 579.17140 | 579.17180 | −0.40 | 116.0382(13), 117.0445 (1), 125.0248 (10), 151.0035 (2), 167.0345 (28), 179.0413 (1), 201.1035 (3), 203.0823 (31), 204.0835 (4), 245.0924(100), 246.0951(19), 247.0961 (2), 271.0607 (4), 289.0706 (47) | / | / | 0.19 ± 0.01 |
| 42a | Europetin c | 9.37 | C16H11O8− | 331.04594 | 331.04602 | −0.08 | 110.0017(37), 111.0082 (6), 121.0299(14), 137.9962(20), 139.0037 (26), 140.0085 (4), 165.9906 (100), 166.9962(24), 181.0143 (11), 193.9856 (6), 243.0284 (5), 271.0239 (8), 287.0173 (5), 316.0210 32), 317.0235 (7) | 0.04 ± 0.001 | / | / |
| ∑ | 0.04 | 4.77 | 0.19 | |||||||
| ∑∑ | 49.84 | 362.37 | 342.02 | |||||||
| Other detected compounds | ||||||||||
| 43a | Tuberonic acid | 9.63 | C12H17O4− | 225.11270 | 225.11193 | 0.77 | 109.0414(11), 109.0694 (11), 110.0387(100), 111.0439(16), 123.0416 (14), 123.0707 (10), 135.0837(10), 136.0548 (36), 161.0720 (8), 161.1000(9), 163.1125 (21), 179.1085 (8), 181.1220(24), 207.1026 (88), 208.1046 (13) | + | + | - |
| 44a | Methyl jasmonate | 11.53 | C13H19O3− | 223.13340 | 223.13275 | 0.65 | 120.0274(27), 121.0237 (23), 123.1018 (28), 141.8757(21), 142.0382 (32), 142.0750 (25), 143.0676 (100), 143.1133 (57), 143.1413(26), 151.0361 (38), 168.8724 (26), 205.8271(26), 205.8642 (22), 214.9475 (21) | + | + | + |
| No | Compounds Name | RT | Formula | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (% of Base Peaks) | Samples (%) | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| AMJ | RBJ | |||||||||
| Betalains and betaxanthins | ||||||||||
| 45a | Amaranthin | 2.68 | C30H35N2O19+ | 727.18340 | 727.18497 | −1.57 | 389.0989 (100), 551.1524 (7) | 78.22 | - | [41,43,44] |
| 46a | Betalamic acid | 3.77 | C9H10NO5+ | 212.05590 | 212.05630 | −0.40 | 102.0344 (3), 106.0293 (9), 120.0454 (100), 121.0475 (12), 122.0468 (2), 130.0292 (3), 138.0547 (1), 148.0389 (8), 149.0394 (1), 166.0469 (1) | 7.51 | 8.29 | |
| 47a | γ-Aminobutyric acid-betaxanthin | 3.16 | C12H15N2O6+ | 283.09300 | 283.09369 | −0.69 | 102.0341(2),116.0698 (4), 119.0361(3), 136.0610(100), 137.0632(9), 148.0400 (60), 149.0426 (8), 212.0448(2), 237.0866(3), 239.0570 (4), 248.0540 (2), 266.0677 (3), 283.0931 (33), 284.0950 (8) | - | 0.86 | |
| 48a | Isoamaranhthin | 4.91 | C30H35N2O19+ | 727.18340 | 727.18378 | −0.38 | 389.0985 (100), 551.1515 (6) | 8.78 | - | |
| 49a | Betanin | 5.11 | C24H27N2O13+ | 551.15130 | 551.15207 | −0.77 | 150.0540 (2), 343.0909 (2), 345.1058 (1), 389.0978(100), 390.0990(32),551.1503 (5) | 3.91 | 70.54 | |
| 50a | Isobetanin | 5.59 | C24H27N2O13+ | 551.15130 | 551.15703 | −5.73 | 150.0531 (2), 343.0895 (1), 389.0959 (100), 390.0999 (25), 391.1010(5), 551.1478 (4), 552.1526 (2) | - | 7.49 | |
| 51a | Decarboxy- dehydro- (iso)amaranthin | 5.86 | C29H33N2O17+ | 681.17790 | 681.18521 | −7.31 | 297.0847 (8), 299.0987 (3), 343.0913 (100), 505.1446 (28) | 1.58 | - | |
| 52a | (2 or 17)- Decarboxy(iso)- betanin | 6.13 | C23H27N2O11+ | 507.16150 | 507.16866 | −7.16 | 150.0535 (2), 151.0613 (1), 299.0993 (2), 345.1059(100), 346.1116 (27), 347.1114 (4), 507.1575 (3) | - | 1.33 | |
| 53a | (2 or 17)- Decarboxy- neobetanin | 6.13 | C23H25N2O11+ | 505.14580 | 505.15438 | −8.58 | 253.0948 (3), 255.0956 (4), 269.0894 (5), 281.0887 (2), 297.0851 (20), 298.0888 (5), 299.0987 (3), 343.0899 (100), 344.0943 (24), 345.1067 (49), 346.1086 (8), 505.1434 (10) | - | 1.53 | |
| 54a | Isoleucine- betaxanthin | 6.94 | C15H21N2O6+ | 325.14000 | 325.14250 | −2.50 | 104.0494(12),106.0621 (17), 119.0612(10), 132.0511(14), 133.0753 (35), 147.0868 (14), 148.0480(12), 150.0540 (16), 173.0704 (14), 189.1365(35), 191.081 (100), 192.0843(17), 205.1273(11), 233.1240 (17), 325.1360 (10) | - | 2.88 | |
| 55a | 6′-O-Feruloyl- betanin | 7.41 | C34H35N2O16+ | 727.19870 | 727.20406 | −5.36 | 389.0975 (100) | - | 7.07 | |
| Total (%) | 100 | 100 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belošević, S.D.; Milinčić, D.D.; Gašić, U.M.; Kostić, A.Ž.; Salević-Jelić, A.S.; Marković, J.M.; Đorđević, V.B.; Lević, S.M.; Pešić, M.B.; Nedović, V.A. Broccoli, Amaranth, and Red Beet Microgreen Juices: The Influence of Cold-Pressing on the Phytochemical Composition and the Antioxidant and Sensory Properties. Foods 2024, 13, 757. https://doi.org/10.3390/foods13050757
Belošević SD, Milinčić DD, Gašić UM, Kostić AŽ, Salević-Jelić AS, Marković JM, Đorđević VB, Lević SM, Pešić MB, Nedović VA. Broccoli, Amaranth, and Red Beet Microgreen Juices: The Influence of Cold-Pressing on the Phytochemical Composition and the Antioxidant and Sensory Properties. Foods. 2024; 13(5):757. https://doi.org/10.3390/foods13050757
Chicago/Turabian StyleBelošević, Spasoje D., Danijel D. Milinčić, Uroš M. Gašić, Aleksandar Ž. Kostić, Ana S. Salević-Jelić, Jovana M. Marković, Verica B. Đorđević, Steva M. Lević, Mirjana B. Pešić, and Viktor A. Nedović. 2024. "Broccoli, Amaranth, and Red Beet Microgreen Juices: The Influence of Cold-Pressing on the Phytochemical Composition and the Antioxidant and Sensory Properties" Foods 13, no. 5: 757. https://doi.org/10.3390/foods13050757
APA StyleBelošević, S. D., Milinčić, D. D., Gašić, U. M., Kostić, A. Ž., Salević-Jelić, A. S., Marković, J. M., Đorđević, V. B., Lević, S. M., Pešić, M. B., & Nedović, V. A. (2024). Broccoli, Amaranth, and Red Beet Microgreen Juices: The Influence of Cold-Pressing on the Phytochemical Composition and the Antioxidant and Sensory Properties. Foods, 13(5), 757. https://doi.org/10.3390/foods13050757