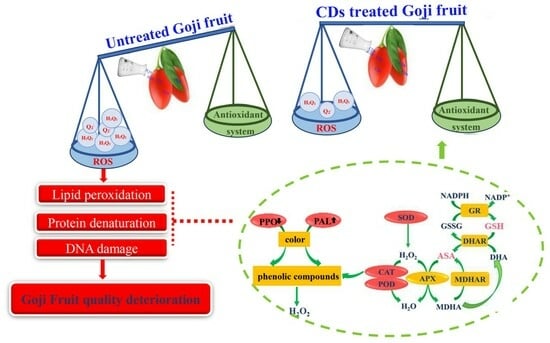
Carbon Dot-Mediated Photodynamic Treatment Improves the Quality Attributes of Post-Harvest Goji Berries (Lycium barbarum L.) via Regulating the Antioxidant System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Carbon Dots (CDs)
2.2. Cytotoxicity of Carbon Dots (CDs)
2.3. Experimental Design and Sample Treatment
2.4. Determination of Physical Properties
2.4.1. Determination of Rotting Rate and Weight Loss Rate of Fresh Goji Berries
2.4.2. Determination of Hardness of Fresh Goji Berries
2.4.3. Determination of Color of Fresh Goji Berries
2.5. Detection of Membrane Permeability and Active Oxygen Metabolism of Fresh Goji Berries
2.5.1. Determination of Electrolyte Permeability
2.5.2. Determination of Malondialdehyde (MDA)
2.5.3. Determination of Hydrogen Peroxide (H2O2) Content and Superoxide Anion (O2•−) Production Rate
2.6. Determination of Quality in Fresh Goji Berries during Storage
2.6.1. Determination of Total Soluble Solids (TSS), Titratable Acidity (TA), and Total Soluble Solids-to-Titratable Acidity Ratio (TSS/TA)
2.6.2. Determination of Soluble Protein Content in Fresh Goji Berries during Storage
2.6.3. Determination of Total Phenol Content, Flavonoid Content, Total Polysaccharide Content, and Betaine Content in Fresh Goji Berries during Storage
2.6.4. Determination of Carotenoid Content in Fresh Goji Berries during Storage
2.7. Determination of Antioxidant Enzyme Activity and ASA–GSH Circulating System
2.7.1. Determination of Antioxidant Enzyme Activity
2.7.2. Determination of Ascorbate–Glutathione Cycle
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Carbon Dots (CDs)
3.2. Optimal Treatment: Carbon Dot-Mediated Photodynamic Treatment of Fresh Goji Berries
3.3. Effect of Carbon Dot-Mediated Photodynamic Treatment on the Appearance Changes of Fresh Goji Berries
3.4. Effects of Carbon Dot-Mediated Photodynamic Treatment on Membrane Permeability and Active Oxygen Metabolism of Fresh Goji Berries during Storage
3.5. Effect of Carbon Dot-Mediated Photodynamic Treatment on the Quality Changes of Post-Harvest Fresh Goji Berries during Storage
3.6. Effects of Carbon Dot-Mediated Photodynamic Treatment on the Activity of the Antioxidant Enzyme System and ASA–GSH Circulating System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, R.H.; Zhang, X.X.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Research progress of Lycium barbarum L. as functional food: Phytochemical composition and health benefits. Curr. Opin. Food Sci. 2022, 47, 100871. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Z.J.; Song, C.B.; Ma, W.P.; Cao, S.Q.; Wei, Z.J. Hydrogen sulfide treatment improves quality attributes via regulating the antioxidant system in goji berry (Lycium barbarum L.). Food Chem. 2023, 405 Pt A, 134858. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Ma, Z.M.; Wang, J.J.; Wang, P.; Lu, D.Y.; Deng, S.F.; Lei, H.L.; Gao, Y.F.; Tao, Y.Y. Treatment with exogenous salicylic acid maintains quality, increases bioactive compounds, and enhances the antioxidant capacity of fresh goji (Lycium barbarum L.) fruit during storage. LWT 2021, 140, 110837. [Google Scholar] [CrossRef]
- Elam, E.; Lv, Y.M.; Wang, W.; Thakur, K.; Ma, W.P.; Ni, Z.J.; Wei, Z.J. Effects of nitric oxide on postharvest storage quality of Lycium barbarum fruit. Food Sci. Technol. 2022, 42, e84122. [Google Scholar] [CrossRef]
- Ban, Z.J.; Wei, W.W.; Yang, X.Z.; Feng, J.H.; Guan, J.F.; Li, L. Combination of heat treatment and chitosan coating to improve postharvest quality of wolfberry (Lycium barbarum). Int. J. Food. Sci. Technol. 2015, 50, 1019–1025. [Google Scholar] [CrossRef]
- Fan, X.J.; Zhang, B.; Yan, H.; Feng, J.T.; Ma, Z.Q.; Zhang, X. Effect of lotus leaf extract incorporated composite coating on the postharvest quality of fresh goji (Lycium barbarum L.) fruit. Physiol. Plant 2019, 148, 132–140. [Google Scholar] [CrossRef]
- Liu, D.; Gu, W.M.; Wang, L.; Sun, J.X. Photodynamic inactivation and its application in food preservation. Crit. Rev. Food. Sci. Nutr. 2023, 63, 2042–2056. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Lukseviciūtė, V.; Kokstaite, R.; Labeikyte, D.; Kaziukonyte, L.; Luksiene, Z. Inactivation of Gram (-) bacteria Salmonella enterica by chlorophyllin-based photosensitization: Mechanism of action and new strategies to enhance the inactivation efficiency. J. Photochem. Photobiol. B 2017, 172, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.L.; Baptista, M.S. Mechanisms of photosensitized lipid oxidation and membrane permeabilization. ACS Omega 2019, 4, 21636–21646. [Google Scholar] [CrossRef]
- Sun, Y.N.; Zhang, M.; Bhandari, B.; Yang, C.H. Recent development of carbon quantum dots: Biological toxicity, antibacterial properties and application in foods. Food Rev. Int. 2022, 38, 1513–1532. [Google Scholar] [CrossRef]
- Maisch, T.; Eichner, A.; Späth, A.; Gollmer, A.; König, B.; Regensburger, J.; Bäumler, W. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PLoS ONE 2014, 9, e111792. [Google Scholar] [CrossRef] [PubMed]
- Penha, C.B.; Bonin, E.; da Silva, A.F.; Hioka, N.; Zanqueta, É.B.; Nakamura, T.U.; de Abreu Filho, B.A.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G. Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT 2017, 76, 198–202. [Google Scholar] [CrossRef]
- Aponiene, K.; Paskeviciute, E.; Reklaitis, I.; Luksiene, Z. Reduction of microbial contamination of fruits and vegetables by hypericin-based photosensitization: Comparison with other emerging antimicrobial treatments. J. Food Eng. 2015, 144, 29–35. [Google Scholar] [CrossRef]
- Ghate, V.S.; Zhou, W.B.; Yuk, H.G. Perspectives and Trends in the Application of Photodynamic Inactivation for Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2019, 18, 402–424. [Google Scholar] [CrossRef]
- Liu, S.H.; Cui, J.L.; Huang, J.B.; Tian, B.S.; Jia, F.; Wang, Z.L. Facile one-pot synthesis of highly fluorescent nitrogen-doped carbon dots by mild hydrothermal method and their applications in detection of Cr(VI) ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 65–71. [Google Scholar] [CrossRef]
- Wen, F.Z.; Li, P.Y.; Yan, H.J.; Su, W. Turmeric carbon quantum dots enhanced chitosan nanocomposite films based on photodynamic inactivation technology for antibacterial food packaging. Carbohydr. Polym. 2023, 311, 120784. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Zhao, Y.N.; Zhang, Y.Y. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II) ion detection. Sens. Actuators B Chem. 2014, 196, 647–652. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.F.; Chen, S. Amphiphilic egg-derived carbon dots: Rapid plasma fabrication, pyrolysis process, and multicolor printing patterns. Angew. Chem. Int. Edit. 2012, 51, 9297–9301. [Google Scholar] [CrossRef]
- Song, Y.B.; Zhu, S.J.; Zhang, S.T.; Fu, Y.Y.; Wang, L.; Zhao, X.H.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Zhai, X.Y.; Zhang, P.; Liu, C.J.; Bai, T.; Li, W.C.; Dai, L.M.; Liu, W.G. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. ChemComm 2012, 48, 7955–7957. [Google Scholar] [CrossRef]
- Wang, B.G.; Tang, W.W.; Lu, H.S.; Huang, Z.Y. Hydrothermal synthesis of ionic liquid-capped carbon quantum dots with high thermal stability and anion responsiveness. J. Mater. Sci. 2015, 50, 5411–5418. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhong, X.; Pan, Q.; Zhang, Y.; Deng, W.T.; Zou, G.Q.; Hou, H.S.; Ji, X.B. A review of carbon dots in synthesis strategy. Coord. Chem. Rev. 2024, 498, 215468. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Fan, D.C.; Jiang, F.J. Effect of carbon dots with chitosan coating on microorganisms and storage quality of modified-atmosphere-packaged fresh-cut cucumber. J. Sci. Food. Agric. 2019, 99, 6032–6041. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Z.; Rhim, J.-W.; Bagheri, R.; Pircheraghi, G.; Lotfali, E. Carboxymethyl cellulose-based functional film integrated with chitosan-based carbon quantum dots for active food packaging applications. Prog. Org. Coat. 2022, 166, 106794. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Hu, J.S.; Wei, H.; Liu, X.Y.; Li, E.S.; Yang, S.H. Rational design of novel carbon-oxygen quantum dots for ratiometrically mapping pH and reactive oxygen species scavenging. Carbon 2022, 190, 115–124. [Google Scholar] [CrossRef]
- Zhu, S.J.; Meng, Q.N.; Wang, L.; Zhang, J.H.; Song, Y.B.; Jin, H.; Zhang, K.; Sun, H.C.; Wang, H.Y.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Edit. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.H.; Ni, Z.J.; Zhang, F.; Zhang, Y.Y.; Liu, M.M.; Thakur, K.; Zhang, J.G.; Wang, S.; Wei, Z.J. 6-Shogaol mediated ROS production and apoptosis via endoplasmic reticulum and mitochondrial pathways in human endometrial carcinoma Ishikawa cells. J. Funct. 2020, 74, 104178. [Google Scholar] [CrossRef]
- Lv, Y.M.; Elnur, E.; Wang, W.; Thakur, K.; Du, J.; Li, H.N.; Ma, W.P.; Liu, Y.Q.; Ni, Z.J.; Wei, Z.J. Hydrogen sulfide treatment increases the antioxidant capacity of fresh Lingwu Long Jujube (Ziziphus jujuba cv. Mill) fruit during storage. Curr. Res. Food Sci. 2022, 5, 949–957. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Y.X.; Yang, S.H.; Li, C.; Li, L.; Gao, S.Y.; Wu, Z.X. Mechanism of ozone treatment in delayed softening of fresh-cut kiwifruit during storage. Postharvest Biol. Technol. 2023, 204, 112469. [Google Scholar] [CrossRef]
- Shi, J.K.; Xie, W.X.; Li, S.N.; Wang, Y.; Wang, Q.G.; Li, Q.Q. Prohibitin StPHB3 affects the browning of fresh-cut potatoes via influencing antioxidant capacity and polyphenol oxidase activation. Postharvest Biol. Technol. 2024, 207, 112598. [Google Scholar] [CrossRef]
- Ding, X.C.; Liu, S.; Duan, X.W.; Pan, X.J.; Dong, B.Y. MAPK cascade and ROS metabolism are involved in GABA-induced disease resistance in red pitaya fruit. Postharvest Biol. Technol. 2023, 200, 112324. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Kim, M.J.; Bang, W.S.; Yuk, H.G. 405 ± 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol. 2017, 62, 124–132. [Google Scholar] [CrossRef]
- Niu, X.D.; Liu, L.; Wang, H.S.; Lin, L.; Yang, Y.A.; Gao, Y.W.; Wang, X.Y.; Sun, J.; Dong, B. Discovery of novel photosensitized nanoparticles as a preservative for the storage of strawberries and their activity against Botrytis cinerea. LWT 2021, 145, 111359. [Google Scholar] [CrossRef]
- Knoblauch, R.; Geddes, C.D. Carbon nanodots in photodynamic antimicrobial therapy: A review. Materials 2020, 13, 4004. [Google Scholar] [CrossRef]
- Chea, S.; Yu, D.J.; Park, J.; Oh, H.D.; Chung, S.W.; Lee, H.J. Fruit softening correlates with enzymatic and compositional changes in fruit cell wall during ripening in ‘Bluecrop’ highbush blueberries. Sci. Hortic. 2019, 245, 163–170. [Google Scholar] [CrossRef]
- Du, F.F.; Shuang, S.M.; Guo, Z.H.; Gong, X.J.; Dong, C.; Xian, M.; Yang, Z.H. Rapid synthesis of multifunctional carbon nanodots as effective antioxidants, antibacterial agents, and quercetin nanoprobes. Talanta 2020, 206, 120243. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.C.; Li, L.; Aghdam, M.S.; Wei, X.X.; Liu, J.Q.; Xu, Y.Q.; Luo, Z.S. Elevated CO2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chem. 2019, 285, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Patsilinakos, A.; Ragno, R.; Carradori, S.; Petralito, S.; Cesa, S. Carotenoid content of Goji berries: CIELAB, HPLC-DAD analyses and quantitative correlation. Food Chem. 2018, 268, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.S.; Tian, S.P.; Zheng, X.L.; Zhou, Z.W.; Xu, Y. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol. Plant 2007, 130, 112–121. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, C.; Xie, J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’ plum fruit. Postharvest Biol. Technol. 2011, 62, 115–120. [Google Scholar] [CrossRef]
- Zhao, Q.X.; Jin, M.J.; Guo, L.Y.; Pei, H.H.; Nan, Y.Y.; Rao, J.P. Modified atmosphere packaging and 1-methylcyclopropene alleviate chilling injury of ‘Youhou’ sweet persimmon during cold storage. Food Packag. Shelf Life 2020, 24, 100479. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Sun, J.Z.; Lin, H.T.; Hung, Y.C.; Zhang, S.; Lin, Y.F.; Lin, T. Paper-based 1-MCP treatment suppresses cell wall metabolism and delays softening of Huanghua pears during storage. J. Sci. Food Agric. 2017, 97, 2547–2552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Li, H.X.; Xi, W.P.; An, W.; Niu, L.L.; Cao, Y.L.; Wang, H.F.; Wang, Y.J.; Yin, Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem. 2015, 173, 718–724. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Jatoi, M.A.; Jurić, S.; Vidrih, R.; Vinceković, M.; Vuković, M.; Jemrić, T. The effects of postharvest application of lecithin to improve storage potential and quality of fresh goji (Lycium barbarum L.) berries. Food Chem. 2017, 230, 241–249. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Huan, C.; Han, S.; Jiang, L.; An, X.J.; Yu, M.L.; Xu, Y.; Ma, R.J.; Yu, Z.F. Postharvest hot air and hot water treatments affect the antioxidant system in peach fruit during refrigerated storage. Postharvest Biol. Technol. 2017, 126, 1–14. [Google Scholar] [CrossRef]
- Stanković, N.K.; Bodik, M.; Šiffalovič, P.; Kotlar, M.; Mičušik, M.; Špitalsky, Z.; Danko, M.; Milivojević, D.D.; Kleinova, A.; Kubat, P.; et al. Antibacterial and antibiofouling properties of light triggered fluorescent hydrophobic carbon quantum dots langmuir–blodgett thin films. ACS Sustain. Chem. Eng. 2018, 6, 4154–4163. [Google Scholar] [CrossRef]
- Nie, X.L.; Jiang, C.Y.; Wu, S.L.; Chen, W.B.F.; Lv, P.F.; Wang, Q.Q.; Liu, J.Y.; Narh, C.; Cao, X.M.; Ghiladi, R.A.; et al. Carbon quantum dots: A bright future as photosensitizers for in vitro antibacterial photodynamic inactivation. J. Photochem. 2020, 206, 111864. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Lu, X.M.; Tang, D.D.; Wu, S.H.; Hou, X.D.; Liu, J.W.; Wu, P. Phosphorescent carbon dots for highly efficient oxygen photosensitization and as photo-oxidative nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef]
- Niazi, Z.; Razavi, F.; Khademi, O.; Aghdam, M.S. Exogenous application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, cv. Karaj) during cold storage. Sci. Hortic. 2021, 285, 110198. [Google Scholar] [CrossRef]
- Ohl, S.; Hedrick, S.A.; Chory, J.; Lamb, C.J. Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell 1990, 2, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Zhang, F.; Tang, Q.J.; Xu, C.S.; Ni, Z.J.; Meng, X.H. Effects of curcumin-based photodynamic treatment on the storage quality of fresh-cut apples. Food Chem. 2019, 274, 415–421. [Google Scholar] [CrossRef]
- Yu, J.S.; Zhang, F.; Zhang, J.; Han, Q.M.; Song, L.L.; Meng, X.H. Effect of photodynamic treatments on quality and antioxidant properties of fresh-cut potatoes. Food Chem. 2021, 362, 130224. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S.; Fard, J.R.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hortic. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Wang, L.J.; Chen, S.J.; Kong, W.F.; Li, S.H.; Archbold, D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006, 41, 244–251. [Google Scholar] [CrossRef]
- Zhu, L.J.; Yu, H.T.; Dai, X.M.; Yu, M.L.; Yu, Z.F. Effect of methyl jasmonate on the quality and antioxidant capacity by modulating ascorbate-glutathione cycle in peach fruit. Sci. Hortic. 2022, 303, 111216. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Ni, Z.-J.; Wang, W.; Thakur, K.; Ma, R.-H.; Ma, W.-P.; Wei, Z.-J. Carbon Dot-Mediated Photodynamic Treatment Improves the Quality Attributes of Post-Harvest Goji Berries (Lycium barbarum L.) via Regulating the Antioxidant System. Foods 2024, 13, 955. https://doi.org/10.3390/foods13060955
Du J, Ni Z-J, Wang W, Thakur K, Ma R-H, Ma W-P, Wei Z-J. Carbon Dot-Mediated Photodynamic Treatment Improves the Quality Attributes of Post-Harvest Goji Berries (Lycium barbarum L.) via Regulating the Antioxidant System. Foods. 2024; 13(6):955. https://doi.org/10.3390/foods13060955
Chicago/Turabian StyleDu, Juan, Zhi-Jing Ni, Wei Wang, Kiran Thakur, Run-Hui Ma, Wen-Ping Ma, and Zhao-Jun Wei. 2024. "Carbon Dot-Mediated Photodynamic Treatment Improves the Quality Attributes of Post-Harvest Goji Berries (Lycium barbarum L.) via Regulating the Antioxidant System" Foods 13, no. 6: 955. https://doi.org/10.3390/foods13060955
APA StyleDu, J., Ni, Z. -J., Wang, W., Thakur, K., Ma, R. -H., Ma, W. -P., & Wei, Z. -J. (2024). Carbon Dot-Mediated Photodynamic Treatment Improves the Quality Attributes of Post-Harvest Goji Berries (Lycium barbarum L.) via Regulating the Antioxidant System. Foods, 13(6), 955. https://doi.org/10.3390/foods13060955