Disclosing the Functional Potency of Three Oxygenated Monoterpenes in Combating Microbial Pathogenesis: From Targeting Virulence Factors to Chicken Meat Preservation
Abstract
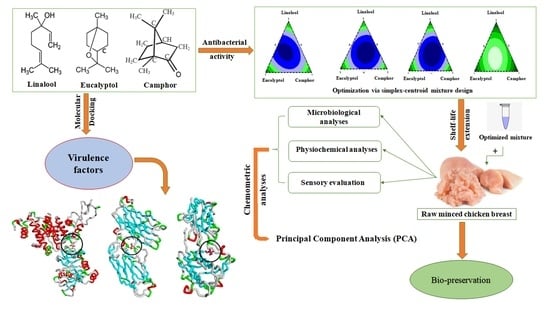
:1. Introduction
2. Materials and Methods
2.1. Bioactive Compounds
2.2. Antibacterial Assays
2.2.1. Bacterial Strains
2.2.2. Determination of Minimum Inhibitory Concentration (MIC)
2.3. Mixture Design
2.4. In Silico Study of the Antibacterial Properties of the Studied Bioactive Compounds
2.4.1. Ligands Preparation
2.4.2. Bacterial Targets Selection and Binding Site Prediction
2.4.3. Molecular Docking Simulations and Interaction Profiles Visualization
2.5. Analysis of Raw Chicken Breast Meat Samples
2.5.1. Samples Preparation
2.5.2. Microbiological Analyses
2.5.3. Physiochemical Analyses
pH Analysis
Evaluation of Protein/Lipid Oxidation
Assessment of Sensory Attributes
2.6. Statistical Analyses
3. Results and Discussion
3.1. Mixture Design
3.1.1. Effect of the Mixture on the Antibacterial Activities
- -
- Anti-S. aureus Activity
- -
- Anti-S. enterica Typhimurium Activity
- -
- Anti-E. coli Activity
- -
- Anti-L. monocytogenes Activity
3.1.2. Mixture Design Optimization
3.2. In Silico Evaluation of the Antibacterial Potential of L, E, and C
3.3. Application of Triple Combination of L, E, and C on Raw Minced Chicken Breast Shelf life
3.3.1. Microbiological Analysis
3.3.2. Physiochemical Analysis
pH Values
Evaluation of Lipid/Protein Oxidation
3.3.3. Sensory Evaluation
3.3.4. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Özlü, H.; Çevik, B.; Atasever, M.; Sarialioğlu, M.; Polat, B. Investigation of meat species adulteration in beef-based meat products via real-time PCR in Türkiye. Qual. Assur. Saf. Crop. Foods 2023, 15, 42–48. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.K.; Venkitanarayana, K. Chapter 17-Spoilage bacteria and meat quality. In Meat Quality Analysis; Biswas, A.K., Mandal, P.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 307–334. [Google Scholar] [CrossRef]
- Filipčev, B. Chapter 16-The effects of aromatic plants and their extracts in food products. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 279–294. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Qamar, M.; Sestili, P.; Saeed, W.; Azeem, M.; Esatbeyoglu, T. Antioxidant Effect of Ocimum basilicum Essential Oil and Its Effect on Cooking Qualities of Supplemented Chicken Nuggets. Antioxidants 2022, 11, 1882. [Google Scholar] [CrossRef]
- Popa, C.L.; Lupitu, A.; Mot, M.D.; Copolovici, L.; Moisa, C.; Copolovici, D.M. Chemical and Biochemical Characterization of Essential Oils and Their Corresponding Hydrolats from Six Species of the Lamiaceae Family. Plants 2021, 10, 2489. [Google Scholar] [CrossRef]
- Tian, Z.; Luo, Q.; Zuo, Z. Seasonal emission of monoterpenes from four chemotypes of Cinnamomum camphora. Ind. Crop. Prod. 2021, 163, 113327. [Google Scholar] [CrossRef]
- Rawat, A.; Rawat, M.; Prakash, O.; Kumar, R.; Punetha, H.; Rawat, D.S. Comparative study on eucalyptol and camphor rich essential oils from rhizomes of Hedychium spicatum Sm. and their pharmacological, antioxidant and antifungal activities. An. Acad. Bras. Ciênc 2022, 94, e20210932. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A. Toxicity of selected monoterpenes and essential oils rich in these compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef] [PubMed]
- Bhowal, M.; Gopal, M. Eucalyptol: Safety and pharmacological profile. J. Pharm. Sci. 2015, 5, 125–131. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.N.; Li, X.; Fan, G.; Qu, S.S.; Song, Y.; Pan, S.Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Hou, Z.; Yang, Q.; Cui, L.; Lu, H.; Li, R.; Liu, Y.; Zhang, Y.; Chen, Y. Antimicrobial mechanism and biocontrol effect of Bacillus cereus XZ30-2 on Aspergillus niger. Qual. Assur. Saf. Crop. Foods 2023, 15, 77–88. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Paulino, B.N.; Silva, G.N.; Araujo, F.F.; Neri-Numa, I.A.; Pastore, G.M.; Bicas, J.L.; Molina, G. Beyond natural aromas: The bioactive and technological potential of monoterpenes. Trends Food Sci. Technol. 2022, 128, 188–201. [Google Scholar] [CrossRef]
- Presenza, L.; Fabrício, L.F.d.F.; Galvão, J.A.; Vieira, T.M.F.d.S. Simplex-centroid mixture design as a tool to evaluate the effect of added flours for optimizing the formulation of native Brazilian freshwater fish burger. LWT 2022, 156, 113008. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Synergistic activities of gaseous oregano and thyme thymol essential oils against Listeria monocytogenes on surfaces of a laboratory medium and radish sprouts. Food Microbiol. 2020, 86, 103357. [Google Scholar] [CrossRef] [PubMed]
- Chaari, M.; Elhadef, K.; Akermi, S.; Hlima, H.B.; Fourati, M.; Mtibaa, A.C.; Ennouri, M.; D’amore, T.; Ali, D.S.; Mousavi Khaneghah, A.; et al. Potentials of beetroot (Beta vulgaris L.) peel extract for quality enhancement of refrigerated beef meat. Qual. Assur. Saf. Crop. Foods 2023, 15, 99–115. [Google Scholar] [CrossRef]
- Watkins, K.; Unnikrishnan, M. Chapter 9-New strategies and targets for antibacterial discovery. In Drug Discovery Targeting Drug-Resistant Bacteria; Kesharwani, P., Chopra, S., Dasgupta, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 249–272. [Google Scholar] [CrossRef]
- Vila, J.; Moreno-Morales, J.; Ballesté-Delpierre, C. Current landscape in the discovery of novel antibacterial agents. Clin. Microbiol. Infect. 2020, 26, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Limon, A.; Aguilar-Toalá, J.E.; Liceaga, A.M. Integration of Molecular Docking Analysis and Molecular Dynamics Simulations for Studying Food Proteins and Bioactive Peptides. J. Agric. Food Chem. 2022, 70, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Asiamah, I.; Obiri, S.A.; Tamekloe, W.; Armah, F.A.; Borquaye, L.S. Applications of molecular docking in natural products-based drug discovery. Sci. Afr. 2023, 20, e01593. [Google Scholar] [CrossRef]
- Elhadef, K.; Akermi, S.; Ben Hlima, H.; Ennouri, K.; Fourati, M.; Ben Braïek, O.; Mellouli, L.; Smaoui, S. Tunisian Pistachio Hull Extracts: Phytochemical Content, Antioxidant Activity, and Foodborne Pathogen Inhibition. J. Food Qual. 2021, 2021, 9953545. [Google Scholar] [CrossRef]
- Mohamadi, N.; Meraghni, M.; Meradci, F.; Necib, A.; El Arbi, M.; Elhadef, K.; Smaoui, S.; Bouaziz, M. Investigation and quantification of the potential antioxidant, inflammatory, and antibacterial bioactive molecules of the extracts of Algerian black and green table olive brine. Qual. Assur. Saf. Crop. Foods 2023, 15, 92–106. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Chaari, M.; Elhadef, K.; Akermi, S.; Hlima, H.B.; Fourati, M.; Chakchouk Mtibaa, A.; Sarkar, T.; Shariati, M.A.; Rebezov, M.; D’Amore, T.; et al. Multiobjective response and chemometric approaches to enhance the phytochemicals and biological activities of beetroot leaves: An unexploited organic waste. Biomass Conv. Bioref. 2022, 13, 15067–15081. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 26 December 2022).
- Available online: http://www.molecular-networks.com/online_demos/corina_demo (accessed on 26 December 2022).
- Available online: https://www.uniprot.org/ (accessed on 26 December 2022).
- Available online: https://swissmodel.expasy.org (accessed on 28 December 2022).
- Available online: http://www.ebi.ac.uk/thornton-srv/databases/ProFunc/ (accessed on 28 December 2022).
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Akermi, S.; Smaoui, S.; Elhadef, K.; Fourati, M.; Louhichi, N.; Chaari, M.; Chakchouk Mtibaa, A.; Baanannou, A.; Masmoudi, S.; Mellouli, L. Cupressus sempervirens Essential Oil: Exploring the Antibacterial Multitarget Mechanisms, Chemcomputational Toxicity Prediction, and Safety Assessment in Zebrafish Embryos. Molecules 2022, 27, 2630. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- U.S. Code of Federal Regulations. 9 CFR Sec. 319.141. 2013. Available online: https://www.ecfr.gov/current/title-9 (accessed on 18 February 2023).
- U.S. Code of Federal Regulations. 9 CFR Sec. 424.4. 2013. Available online: https://www.ecfr.gov/current/title-9 (accessed on 18 February 2023).
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the US: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef]
- Schilling, M.W.; Pham, A.J.; Williams, J.B.; Xiong, Y.L.; Dhowlaghar, N.; Tolentino, A.C.; Kin, S. Changes in the physiochemical, microbial, and sensory characteristics of fresh pork sausage containing rosemary and green tea extracts during retail display. Meat Sci. 2018, 143, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Turgut, S.S.; Işıkçı, F.; Soyer, A. Antioxidant activity of pomegranate peel extract on lipid and protein oxidation in beef meatballs during frozen storage. Meat Sci. 2017, 129, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Ben Hlima, H.; Ghorbel, R. The effect of sodium lactate and lactic acid combinations on the microbial, sensory, and chemical attributes of marinated chicken thigh. Poult. Sci. 2012, 91, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-1:2013; International Organization for Standardization Microbiology of the Food Chain—Horizontal method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 17410; International Organization for Standardization, I. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 21528-2. 20; Microbiology of Food and Animal Feeding Stuffs–Horizontal Methods for the Detection and Enumeration of Enterobacteriaceae. ISO: Geneva, Switzerland, 2004.
- Mukhametov, A.; Chulenyov, A.; Kazak, A.; Semenycheva, I. Physicochemical and microbiological analysis of goose meat. Qual. Assur. Saf. Crop. Foods 2023, 15, 49–58. [Google Scholar] [CrossRef]
- Elhadef, K.; Smaoui, S.; Ben Hlima, H.; Ennouri, K.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Mellouli, L. Effects of Ephedra alata extract on the quality of minced beef meat during refrigerated storage: A chemometric approach. Meat Sci. 2020, 170, 108246. [Google Scholar] [CrossRef] [PubMed]
- Cagdas, E.; Kumcuoglu, S. Effect of grape seed powder on oxidative stability of precooked chicken nuggets during frozen storage. J. Food Sci. Technol. 2015, 52, 2918–2925. [Google Scholar] [CrossRef]
- Mtibaa, A.C.; Smaoui, S.; Ben Hlima, H.; Sellem, I.; Ennouri, K.; Mellouli, L. Enterocin BacFL31 from a Safety Enterococcus faecium FL31: Natural Preservative Agent Used Alone and in Combination with Aqueous Peel Onion (Allium cepa) Extract in Ground Beef Meat Storage. BioMed Res. Int. 2019, 2019, e4094890. [Google Scholar] [CrossRef]
- Batool, T.; Farooq, S.; Roohi, N.; Mahmud, A.; Usman, M.; Ghayas, A.; Ahmad, S. Yerel Aseel Tavuklarında Farklı Miktarlarda Diyetsel Lizin Uygulamalarının Et Kalite Özelliklerine Etkisi. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 639–645. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Mechchate, H.; Moja, S.; Baudino, S.; Saleh, A.; Al Kamaly, O.M.; Grafov, A.; Greche, H. Optimized Antibacterial Effects in a Designed Mixture of Essential Oils of Myrtus communis, Artemisia herba-alba and Thymus serpyllum for Wide Range of Applications. Foods 2022, 11, 132. [Google Scholar] [CrossRef]
- Karaca, N.; Şener, G.; Demirci, B.; Demirci, F. Synergistic antibacterial combination of Lavandula latifolia Medik. essential oil with camphor. Z. Für. Naturforschung 2021, 76, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Bahadirli, N.P. Comparison of Chemical Composition and Antimicrobial Activity of Salvia fruticosa Mill. and S. aramiensis Rech. Fill.(Lamiaceae). J. Essent. Oil-Bear. Plants 2022, 25, 716–727. [Google Scholar] [CrossRef]
- Tardugno, R.; Serio, A.; Pellati, F.; D’Amato, S.; Chaves López, C.; Bellardi, M.G.; Di Vito, M.; Savini, V.; Paparella, A.; Benvenuti, S. Lavandula x intermedia and Lavandula angustifolia essential oils: Phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2019, 33, 3330–3335. [Google Scholar] [CrossRef]
- Rodrigues, M.; Lopes, A.C.; Vaz, F.; Filipe, M.; Alves, G.; Ribeiro, M.P.; Coutinho, P.; Araujo, A.R.T.S. Thymus mastichina: Composition and Biological Properties with a Focus on Antimicrobial Activity. Pharmaceuticals 2020, 13, 479. [Google Scholar] [CrossRef]
- Ghaly, M.F.; Nasr, Z.M.; Abousaty, A.I.; Seadawy, H.G.; Shaheen, M.A.A.; Albogami, S.; Al-Sanea, M.M.; Bendary, M.M. Alternative and Complementary Therapies against Foodborne Salmonella Infections. Antibiotics 2021, 10, 1453. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Puvača, N.; Milenković, J.; Galonja Coghill, T.; Bursić, V.; Petrović, A.; Tanasković, S.; Miljković, T. Antimicrobial activity of selected essential oils against selected pathogenic bacteria: In vitro study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef]
- Nafis, A.; Ouedrhiri, W.; Iriti, M.; Mezrioui, N.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Hassani, L. Chemical composition and synergistic effect of three Moroccan lavender EOs with ciprofloxacin against foodborne bacteria: A promising approach to modulate antimicrobial resistance. Lett. Appl. Microbiol. 2021, 72, 698–705. [Google Scholar] [CrossRef]
- Maurya, A.; Singh, V.K.; Das, S.; Prasad, J.; Kedia, A.; Upadhyay, N.; Dubey, N.K.; Dwivedy, A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy Against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Front. Microbiol. 2021, 12, 751062. [Google Scholar] [CrossRef]
- Wallock-Richards, D.J.; Marles-Wright, J.; Maitra, D.J.; Clarke, A.; Dodds, M.; Hanley, B.; Campopiano, D.J. Molecular basis of Streptococcus mutans sortase A inhibition by the flavonoid natural product trans-chalcone. ChemComm 2015, 51, 10483–10485. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, L.; Zhao, Y.; Lanzi, G.; Wang, X.; Zhang, C.; Guan, J.; Wang, W.; Guo, X.; Meng, Y.; et al. Hibifolin, a Natural Sortase A Inhibitor, Attenuates the Pathogenicity of Staphylococcus aureus and Enhances the Antibacterial Activity of Cefotaxime. Microbiol. Spectr. 2022, 10, e00950-22. [Google Scholar] [CrossRef] [PubMed]
- Akter, T.; Chakma, M.; Tanzina, A.Y.; Rumi, M.H.; Shimu, M.S.S.; Saleh, M.A.; Mahmud, S.; Sami, S.A.; Emran, T.B. Curcumin Analogues as a Potential Drug against Antibiotic Resistant Protein, β-Lactamases and L, D-Transpeptidases Involved in Toxin Secretion in Salmonella typhi: A Computational Approach. BioMedInformatics 2022, 2, 77–100. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Teng, Z.; Zhou, X.; Wang, X.; Zhang, B.; Lu, G.; Niu, X.; Yang, Y.; Deng, X. Phloretin Attenuates Listeria monocytogenes Virulence Both In vitro and In vivo by Simultaneously Targeting Listeriolysin O and Sortase A. Front. Cell. Infect. Microbiol. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.C.; Sahoo, S.P.; Mishra, R.R. Insilico analysis of Listeriolysin-O with its natural inhibitors. J. Entomol. Zool. Stud. 2018, 6, 1987–1990. [Google Scholar]
- Saha, S.B.; Verma, V. In Silico analysis of Escherichia coli polyphosphate kinase (PPK) as a novel antimicrobial drug target and its high throughput virtual screening against PubChem library. Bioinformation 2013, 9, 518–523. [Google Scholar] [CrossRef]
- Diass, K.; Merzouki, M.; Elfazazi, K.; Azzouzi, H.; Challioui, A.; Azzaoui, K.; Rhazi, L. Essential oil of lavandula officinalis: Chemical composition and antibacterial activities. Plants 2023, 12, 1571. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Švarc-Gajić, J.; Elhadef, K.; Ben Saad, R.; Brini, F.; Mnif, W.; Ben Hsouna, A. The essential oil of tunisian halophyte Lobularia maritima: A natural food preservative agent of ground beef meat. Life 2022, 12, 1571. [Google Scholar] [CrossRef]
- Smaoui, S.; Hsouna, A.B.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Mellouli, L. Bio-preservative effect of the essential oil of the endemic Mentha piperita used alone and in combination with BacTN635 in stored minced beef meat. Meat Sci. 2016, 117, 196–204. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Davis, M.L.; Johnson, M.G.; Ricke, S.C. Chapter 13-Listeria monocytogenes Adaptation and Growth at Low Temperatures: Mechanisms and Implications for Foodborne Disease. In Food and Feed Safety Systems and Analysis; Ricke, S.C., Atungulu, G.G., Rainwater, C.E., Park, S.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 227–248. [Google Scholar] [CrossRef]
- Afnor, N. Hygiene and Safety Foods-Validation of the Microbiological Shelf Life-Perishable and Cooled Foods; AFNOR: La Plaine-saint-denis, France, 2004. [Google Scholar]
- Peighambardoust, S.H.; Yaghoubi, M.; Hosseinpour, A.; Alirezalu, K.; Soltanzadeh, M.; Dadpour, M. Development and Application of Dual-Sensors Label in Combination with Active Chitosan-Based Coating Incorporating Yarrow Essential Oil for Freshness Monitoring and Shelf-Life Extension of Chicken Fillet. Foods 2022, 11, 3533. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobiş, O.; Lassoued, M.A. 1, 8-Cineol (Eucalyptol) Disrupts Membrane Integrity and Induces Oxidative Stress in Methicillin-Resistant Staphylococcus aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Chen, W.; Chen, H.; Zhong, Q.; Zhang, H.; Zhang, M.; Chen, W. Antibacterial mechanism of linalool against L. monocytogenes, a metabolomic study. Food Control 2022, 132, 108533. [Google Scholar] [CrossRef]
- Septama, A.W.; Tasfiyati, A.N.; Rahmi, E.P.; Jantan, I.; Dewi, R.T.; Jaisi, A. Antibacterial, bacteriolytic, and antibiofilm activities of the essential oil of temu giring (Curcuma heyneana Val.) against foodborne pathogens. Food Sci. Technol. Int. 2023, 2023, 10820132231178060. [Google Scholar] [CrossRef] [PubMed]
- Dudnyk, I.; Janeček, E.-R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.E.; Saracila, M. In vitro antioxidant properties of berry leaves and their inhibitory effect on lipid peroxidation of thigh meat from broiler chickens. Eur. J. Lipid Sci. Technol. 2020, 122, 1900384. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant by-product antioxidants: Control of protein-lipid oxidation in meat and meat products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Sujiwo, J.; Kim, D.; Jang, A. Relation among quality traits of chicken breast meat during cold storage: Correlations between freshness traits and torrymeter values. Poult. Sci. 2018, 97, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; Silva, M.V.D.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors–a review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Karami, N.; Shavisi, N. Effect of Ziziphora clinopodioides essential oil on shelf life and fate of Listeria monocytogenes and Staphylococcus aureus in refrigerated chicken meatballs. J. Food Saf. 2018, 38, e12394. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Arshad, M.S.; Imran, M.; Imran, A.; Hussain, S. Oxidative stability and lipid oxidation flavoring volatiles in antioxidants treated chicken meat patties during storage. Lipids Health Dis. 2017, 16, 27. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current State of the Art on the Antioxidant Activity of Sage (Salvia spp.) and Its Bioactive Components. Planta Med. 2020, 86, 224–238. [Google Scholar] [CrossRef]







| Bacteria | Targets (Receptors) | Compounds (Ligands) | Free Energy of Binding (Kcal/mol) | Number of Interacting Residues | H-Bond Residues |
|---|---|---|---|---|---|
| S. aureus NCTC 8325 | Sortase A | Linalool | −6.5 | 6 | PRO333 |
| Eucalyptol | −6.3 | 6 | - | ||
| Camphor | −6.3 | 4 | - | ||
| S. enterica Typhimurium ATCC 700720 | YcbB | Linalool | −6.2 | 7 | ASP231 |
| Eucalyptol | −5.9 | 3 | - | ||
| Camphor | −5.9 | 6 | - | ||
| L. monocytogenes strain 10403S | Listeriolysin O | Linalool | −6.1 | 6 | TYR414 |
| Eucalyptol | −5.6 | 3 | - | ||
| Camphor | −5.6 | 5 | - | ||
| E. coli (strain K12) | PPK | Linalool | −6.7 | 8 | LEU467 |
| Eucalyptol | −6.6 | 5 | - | ||
| Camphor | −6.5 | 3 | - |
| Days of Storage | ||||||
|---|---|---|---|---|---|---|
| Samples | 0 | 3 | 7 | 10 | 14 | |
| APC | Control | 3.96 ± 0.05 aA | 4.82 ± 0.17 eB | 5.26 ± 0.13 cC | 6.48 ± 0.22 cD | 7.67 ± 0.25 eE |
| BHT | 3.96 ± 0.05 aA | 4.46 ± 0.12 dB | 4.96 ± 0.12 bC | 5.08 ± 0.19 aC | 5.68 ± 0.17 bD | |
| 1-L/E/C | 3.96 ± 0.05 aA | 4.35 ± 0.15 cB | 4.99 ± 0.09 bC | 5.43 ± 0.15 bD | 6.12 ± 0.21 dE | |
| 1.5-L/E/C | 3.96 ± 0.05 aA | 4.27 ± 0.1 bB | 4.87 ± 0.07 aC | 5.04 ± 0.16 aD | 5.91 ± 0.19 cE | |
| 2-L/E/C | 3.96 ± 0.05 aA | 4.05 ± 0.09 aA | 4.82 ± 0.11 aB | 4.94 ± 0.11 aB | 5.62 ± 0.12 aC | |
| PTC | Control | 3.46 ± 0.02 aA | 4.26 ± 0.13 cB | 5.11 ± 0.15 cC | 6.30 ± 0.19 cD | 7.51 ± 0.23 eE |
| BHT | 3.46 ± 0.02 aA | 3.89 ± 0.1 bB | 4.91 ± 0.12 bC | 4.98 ± 0.12 aC | 5.61 ± 0.11 bD | |
| 1-L/E/C | 3.46 ± 0.02 aA | 3.96 ± 0.06 bcB | 4.94 ± 0.07 bC | 5.38 ± 0.18 bD | 5.96 ± 0.13 dE | |
| 1.5-L/E/C | 3.46 ± 0.02 aA | 3.84 ± 0.08 bB | 4.88 ± 0.09 abC | 4.95 ± 0.1 aC | 5.78 ± 0.15 cD | |
| 2-L/E/C | 3.46 ± 0.02 aA | 3.78 ± 0.11 aA | 4.73 ± 0.08 aB | 4.92 ± 0.09 aB | 5.49 ± 0.1 aC | |
| EC | Control | <1 | 1.45 ± 0.03 dA | 1.954 ± 0.02 dB | 2.14 ± 0.01 dC | 3.20 ± 0.11 cD |
| BHT | <1 | 1.20 ± 0.025 cA | 1.31 ± 0.07 bA | 1.53 ± 0.02 bB | 1.98 ± 0.04 aC | |
| 1-L/E/C | <1 | 1.23 ± 0.027 cA | 1.49 ± 0.04 cB | 1.86 ± 0.025 cC | 2.06 ± 0.06 bD | |
| 1.5-L/E/C | <1 | 1.12 ± 0.01 bA | 1.27 ± 0.03 bB | 1.50 ± 0.03 bC | 1.96 ± 0.03 aD | |
| 2-L/E/C | <1 | 1.02 ± 0.02 aA | 1.14 ± 0.01 aB | 1.43 ± 0.01 aC | 1.90 ± 0.02 aD | |
| Days of Storage | ||||||
|---|---|---|---|---|---|---|
| Samples | 0 | 3 | 7 | 10 | 14 | |
| pH | Control | 5.79 ± 0.12 aA | 6.05 ± 0.15 bB | 6.24 ± 0.13 bC | 6.51 ± 0.16 cD | 6.84 ± 0.17 cE |
| BHT | 5.79 ± 0.12 aA | 5.95 ± 0.17 aB | 5.97 ± 0.19 aB | 6.03 ± 0.17 aB | 6.2 ± 0.13 bC | |
| 1-L/E/C | 5.79 ± 0.12 aA | 6.03 ± 0.24 bB | 6.11 ± 0.26 bB | 6.21 ± 0.14 bC | 6.31 ± 0.14 bD | |
| 1.5-L/E/C | 5.79 ± 0.12 aA | 5.99 ± 0.32 aB | 6.01 ± 0.15 aB | 6.15 ± 0.13 bC | 6.21 ± 0.12 bC | |
| 2-L/E/C | 5.79 ± 0.12 aA | 5.87 ± 0.12 aA | 5.99 ± 0.14 aB | 6.09 ± 0.11 aC | 6.14 ± 0.14 aC | |
| PV | Control | 0.04 ± 0.001 aA | 0.12 ± 0.004 cB | 0.32 ± 0.01 dC | 0.47 ± 0.02 cD | 0.85 ± 0.02 dE |
| BHT | 0.04 ± 0.001 aA | 0.07 ± 0.001 aB | 0.24 ± 0.002 bC | 0.31 ± 0.009 bD | 0.6 ± 0.02 bE | |
| 1-L/E/C | 0.04 ± 0.001 aA | 0.09 ± 0.001 bB | 0.28 ± 0.001 cC | 0.38 ± 0.008 bD | 0.71 ± 0.01 cE | |
| 1.5-L/E/C | 0.04 ± 0.001 aA | 0.08 ± 0.001 bB | 0.23 ± 0.002 bC | 0.34 ± 0.002 bD | 0.65 ± 0.02 bE | |
| 2-L/E/C | 0.04 ± 0.001 aA | 0.06 ± 0.001 aA | 0.19 ± 0.002 aB | 0.28 ± 0.001 aC | 0.43 ± 0.01 aD | |
| CD | Control | 0.51 ± 0.02 aA | 1.27 ± 0.01 eC | 1.89 ± 0.014 dD | 0.85 ± 0.021 dB | 0.76 ± 0.025 cB |
| BHT | 0.51 ± 0.02 aA | 0.97 ± 0.014 dB | 0.99 ± 0.013 bB | 0.57 ± 0.02 bA | 0.51 ± 0.018 bA | |
| 1-L/E/C | 0.51 ± 0.02 aA | 0.82 ± 0.014 cC | 1.28 ± 0.04 cD | 0.66 ± 0.02 cB | 0.61 ± 0.01 cB | |
| 1.5-L/E/C | 0.51 ± 0.02 aA | 0.69 ± 0.012 bB | 1.07 ± 0.01 bC | 0.58 ± 0.01 bB | 0.54 ± 0.02 bA | |
| 2-L/E/C | 0.51 ± 0.02 aA | 0.59 ± 0.01 aA | 0.67 ± 0.02 aC | 0.49 ± 0.02 aB | 0.45 ± 0.01 aB | |
| TBARS | Control | 0.1 ± 0.004 aA | 0.32 ± 0.01 dB | 0.89 ± 0.09 dC | 1.65 ± 0.03 cD | 2.13 ± 0.07 cE |
| BHT | 0.1 ± 0.004 aA | 0.21 ± 0.011 bB | 0.28 ± 0.042 bB | 0.36 ± 0.004 aC | 0.42 ± 0.03 aD | |
| 1-L/E/C | 0.1 ± 0.004 aA | 0.28 ± 0.01 cB | 0.56 ± 0.011 cC | 0.76 ± 0.03 bD | 1.12 ± 0.05 bE | |
| 1.5-L/E/C | 0.1 ± 0.004 aA | 0.24 ± 0.011 bB | 0.30 ± 0.012 bC | 0.34 ± 0.02 aC | 0.4 ± 0.05 aD | |
| 2-L/E/C | 0.1 ± 0.004 aA | 0.14 ± 0.012 aA | 0.22 ± 0.01 aB | 0.30 ± 0.01 aC | 0.38 ± 0.04 aD | |
| Carbonyls | Control | 0.24 ± 0.008 aA | 0.52 ± 0.02 dB | 0.68 ± 0.018 cC | 0.89 ± 0.03 dD | 1.26 ± 0.014 dE |
| BHT | 0.24 ± 0.008 aA | 0.43 ± 0.012 cB | 0.45 ± 0.013 aB | 0.56 ± 0.02 aC | 0.82 ± 0.03 bD | |
| 1-L/E/C | 0.24 ± 0.008 aA | 0.48 ± 0.01 cB | 0.57 ± 0.013 bC | 0.78 ± 0.02 cD | 1.12 ± 0.04 cE | |
| 1.5-L/E/C | 0.24 ± 0.008 aA | 0.36 ± 0.011 bB | 0.49 ± 0.014 aC | 0.73 ± 0.01 cD | 0.89 ± 0.03 bE | |
| 2-L/E/C | 0.24 ± 0.008 aA | 0.29 ± 0.01 aA | 0.43 ± 0.012 aB | 0.65 ± 0.01 bC | 0.69 ± 0.02 aC | |
| Days of Storage | ||||||
|---|---|---|---|---|---|---|
| Samples | 0 | 3 | 7 | 10 | 14 | |
| Appearance | Control | 8.6 ± 0.24 aE | 6.7 ± 0.21 aD | 5.58 ± 0.2 aC | 4.96 ± 0.12 aB | 4.21 ± 0.08 aA |
| BHT | 8.6 ± 0.24 aD | 7.5 ± 0.17 bC | 6.72 ± 0.05 bB | 6.48 ± 0.21 bB | 5.86 ± 0.17 cA | |
| 1-L/E/C | 8.6 ± 0.24 aD | 7.2 ± 0.14 bD | 6.68 ± 0.23 bC | 6.41 ± 0.19 bB | 5.36 ± 0.04 bA | |
| 1.5-L/E/C | 8.6 ± 0.24 aC | 7.5 ± 0.23 bB | 6.78 ± 0.19 dA | 6.72 ± 0.14 bA | 6.58 ± 0.13 bA | |
| 2-L/E/C | 8.6 ± 0.24 aD | 7.79 ± 0.29 cC | 7.6 ± 0.11 bC | 7.08 ± 0.18cB | 5.96 ± 0.11cA | |
| Color | Control | 8.25 ± 0.19 aE | 7.23 ± 0.09 aD | 6.89 ± 0.26 bC | 5.13 ± 0.06 aB | 4.08 ± 0.06 aA |
| BHT | 8.25 ± 0.19 aD | 7.61 ± 0.07 bC | 6.98 ± 0.18 cB | 5.32 ± 0.14 cA | 5.21 ± 0.12 cA | |
| 1-L/E/C | 8.25 ± 0.19 aE | 7.43 ± 0.16 cD | 6.66 ± 0.08 aC | 5.27 ± 0.17 bB | 4.98 ± 0.09 bA | |
| 1.5-L/E/C | 8.25 ± 0.19 aD | 7.58 ± 0.25 cC | 6.83 ± 0.19 bB | 5.36 ± 0.09 cA | 5.17 ± 0.13 cA | |
| 2-L/E/C | 8.25 ± 0.19 aD | 7.62 ± 0.22 bC | 7.12 ± 0.22 dB | 5.47 ± 0.18 dA | 5.38 ± 0.14 dA | |
| Odor | Control | 8.02 ± 0.16 aD | 7.25 ± 0.17 aC | 5.08 ± 0.06 aB | 4.86 ± 0.05 aB | 4.23 ± 0.1 aA |
| BHT | 8.02 ± 0.16 aD | 7.65 ± 0.25 cC | 6.87 ± 0.12 cB | 5.76 ± 0.13 cA | 5.49 ± 0.17 cA | |
| 1-L/E/C | 8.02 ± 0.16 aD | 7.53 ± 0.27 bC | 6.41 ± 0.03 bB | 5.47 ± 0.07 bA | 5.31 ± 0.06 bA | |
| 1.5-L/E/C | 8.02 ± 0.16 aD | 7.68 ± 0.10 cC | 6.81 ± 0.17 cB | 5.69 ± 0.17 cA | 5.45 ± 0.18 cA | |
| 2-L/E/C | 8.02 ± 0.16 aD | 7.84 ± 0.29 dC | 6.96 ± 0.12 dB | 5.94 ± 0.15 dA | 5.72 ± 0.15 dA | |
| Overall acceptability | Control | 8.06 ± 0.11 aE | 7.23 ± 0.20 aD | 6.33 ± 0.09 aC | 5.32 ± 0.08 aB | 4.63 ± 0.08 aA |
| BHT | 8.06 ± 0.11 aD | 7.52 ± 0.13 cC | 6.71 ± 0.14 cB | 5.58 ± 0.16 bBA | 5.12 ± 0.11 cA | |
| 1-L/E/C | 8.06 ± 0.11 aE | 7.46 ± 0.15 bD | 6.58 ± 0.11 bC | 5.48 ± 0.12 bB | 4.96 ± 0.07 bA | |
| 1.5-L/E/C | 8.06 ± 0.11 aD | 7.59 ± 0.21 cC | 6.75 ± 0.23 cB | 5.67 ± 0.12 cBA | 5.23 ± 0.12 dA | |
| 2-L/E/C | 8.06 ± 0.11 aD | 7.73 ± 0.08 dC | 6.86 ± 0.15 dB | 5.79 ± 0.07 cA | 5.47 ± 0.15 eA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akermi, S.; Chaari, M.; Elhadef, K.; Fourati, M.; Chakchouk Mtibaa, A.; Agriopoulou, S.; Smaoui, S.; Mellouli, L. Disclosing the Functional Potency of Three Oxygenated Monoterpenes in Combating Microbial Pathogenesis: From Targeting Virulence Factors to Chicken Meat Preservation. Foods 2024, 13, 965. https://doi.org/10.3390/foods13060965
Akermi S, Chaari M, Elhadef K, Fourati M, Chakchouk Mtibaa A, Agriopoulou S, Smaoui S, Mellouli L. Disclosing the Functional Potency of Three Oxygenated Monoterpenes in Combating Microbial Pathogenesis: From Targeting Virulence Factors to Chicken Meat Preservation. Foods. 2024; 13(6):965. https://doi.org/10.3390/foods13060965
Chicago/Turabian StyleAkermi, Sarra, Moufida Chaari, Khaoula Elhadef, Mariam Fourati, Ahlem Chakchouk Mtibaa, Sofia Agriopoulou, Slim Smaoui, and Lotfi Mellouli. 2024. "Disclosing the Functional Potency of Three Oxygenated Monoterpenes in Combating Microbial Pathogenesis: From Targeting Virulence Factors to Chicken Meat Preservation" Foods 13, no. 6: 965. https://doi.org/10.3390/foods13060965
APA StyleAkermi, S., Chaari, M., Elhadef, K., Fourati, M., Chakchouk Mtibaa, A., Agriopoulou, S., Smaoui, S., & Mellouli, L. (2024). Disclosing the Functional Potency of Three Oxygenated Monoterpenes in Combating Microbial Pathogenesis: From Targeting Virulence Factors to Chicken Meat Preservation. Foods, 13(6), 965. https://doi.org/10.3390/foods13060965