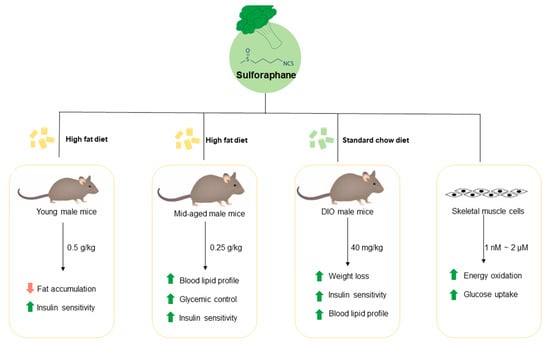
Sulforaphane Ameliorates High-Fat-Diet-Induced Metabolic Abnormalities in Young and Middle-Aged Obese Male Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.1.1. Prevention Studies in Young Mice
2.1.2. Prevention Studies in Middle-Aged Mice
2.1.3. Treatment Studies in Middle-Aged Obese Mice
2.2. Cell Culture
2.2.1. Glucose Uptake Assay
2.2.2. Fatty Acid and Pyruvate Oxidation
2.3. Data Analysis
3. Results
3.1. Long-Term Dietary Supplementation of SFN Ameliorated HFD-Induced Metabolic Abnormalities in Young Male Mice
3.2. Dietary Intake of SFN Enhanced Glucose Homeostasis and Lipid Profile in Middle-Aged Obese Mice
3.3. Dietary Supplementation with SFN Promoted Insulin Sensitivity and Ameliorated Hyperglycemia Induced by HFD
3.4. Treatment with SFN Promotes Weight Loss in Diet-Induced Obese Mice
3.5. Treatment with SFN Promoted Lipid Profile in DIO Male Mice and Enhanced Energy Oxidation In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Centers for Disease Control and Prevention. A Snapshot: Diabetes in the United States. Available online: https://www.cdc.gov/diabetes/library/socialmedia/infographics/diabetes.html (accessed on 27 October 2023).
- International Diabetes Federation. IDF Diabetes Atlas. Available online: https://www.diabetesatlas.org (accessed on 27 October 2023).
- NCD Risk Factor Collaboration. Worldwide Trends in Diabetes Since 1980: A Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Brundisini, F.; Vanstone, M.; Hulan, D.; DeJean, D.; Giacomini, M. Type 2 Diabetes Patients’ and Providers’ Differing Perspectives on Medication Nonadherence: A Qualitative Meta-Synthesis. BMC Health Serv. Res. 2015, 15, 516. [Google Scholar] [CrossRef] [PubMed]
- Stoffers, D.A. The Development of Beta-Cell Mass: Recent Progress and Potential Role of Glp-1. Horm. Metab. Res. 2004, 36, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, H.; Mizukami, H.; Yagihashi, N.; Wada, R.; Hanyu, C.; Yagihashi, S. Reduced Beta-Cell Mass and Expression of Oxidative Stress-Related DNA Damage in the Islet of Japanese Type II Diabetic Patients. Diabetologia 2002, 45, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Del Guerra, S.; Marselli, L.; Lupi, R.; Masini, M.; Pollera, M.; Bugliani, M.; Boggi, U.; Vistoli, F.; Mosca, F.; et al. Pancreatic Islets from Type 2 Diabetic Patients Have Functional Defects and Increased Apoptosis That Are Ameliorated by Metformin. J. Clin. Endocrinol. Metab. 2004, 89, 5535–5541. [Google Scholar] [CrossRef] [PubMed]
- Cozar-Castellano, I.; Fiaschi-Taesch, N.; Bigatel, T.A.; Takane, K.K.; Garcia-Ocana, A.; Vasavada, R.; Stewart, A.F. Molecular Control of Cell Cycle Progression in the Pancreatic Beta-Cell. Endocr. Rev. 2006, 27, 356–370. [Google Scholar] [CrossRef]
- Tourrel, C.; Bailbe, D.; Lacorne, M.; Meile, M.J.; Kergoat, M.; Portha, B. Persistent Improvement of Type 2 Diabetes in the Goto-Kakizaki Rat Model by Expansion of the Beta-Cell Mass During the Prediabetic Period with Glucagon-Like Peptide-1 or Exendin-4. Diabetes 2002, 51, 1443–1452. [Google Scholar] [CrossRef]
- Misra, R.; Fitch, C.; Roberts, D.; Wright, D. Community-Based Diabetes Screening and Risk Assessment in Rural West Virginia. J. Diabetes Res. 2016, 2016, 2456518. [Google Scholar] [CrossRef]
- Lee, Y.R.; Noh, E.M.; Han, J.H.; Kim, J.M.; Hwang, B.M.; Kim, B.S.; Lee, S.H.; Jung, S.H.; Youn, H.J.; Chung, E.Y.; et al. Sulforaphane Controls TPA-Induced MMP-9 Expression through the NF-kappaB Signaling Pathway, but Not AP-1, in MCF-7 Breast Cancer Cells. BMB Rep. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- Suppipat, K.; Park, C.S.; Shen, Y.; Zhu, X.; Lacorazza, H.D. Sulforaphane Induces Cell Cycle Arrest and Apoptosis in Acute Lymphoblastic Leukemia Cells. PLoS ONE 2012, 7, e51251. [Google Scholar] [CrossRef]
- Hahm, E.R.; Chandra-Kuntal, K.; Desai, D.; Amin, S.; Singh, S.V. Notch Activation Is Dispensable for D, L-Sulforaphane-Mediated Inhibition of Human Prostate Cancer Cell Migration. PLoS ONE 2012, 7, e44957. [Google Scholar] [CrossRef]
- de Souza, C.G.; Sattler, J.A.; de Assis, A.M.; Rech, A.; Perry, M.L.; Souza, D.O. Metabolic Effects of Sulforaphane Oral Treatment in Streptozotocin-Diabetic Rats. J. Med. Food 2012, 15, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cui, W.; Xin, Y.; Miao, X.; Barati, M.T.; Zhang, C.; Chen, Q.; Tan, Y.; Cui, T.; Zheng, Y.; et al. Prevention by Sulforaphane of Diabetic Cardiomyopathy Is Associated with Up-Regulation of Nrf2 Expression and Transcription Activation. J. Mol. Cell. Cardiol. 2013, 57, 82–95. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, E.K.; Moon, W.S.; Park, J.W.; Kim, H.J.; So, H.S.; Park, R.; Kwon, K.B.; Park, B.H. Sulforaphane Protects against Cytokine- and Streptozotocin-Induced Beta-Cell Damage by Suppressing the NF-kappaB Pathway. Toxicol. Appl. Pharmacol. 2009, 235, 57–67. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-Dependent Proteasomal Degradation of Transcription Factor Nrf2 Contributes to the Negative Regulation of Antioxidant Response Element-Driven Gene Expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Sun, W.; Tan, Y.; Liu, Y.; Zheng, Y.; Liu, Q.; Cai, L.; Sun, J. Sulforaphane Attenuation of Type 2 Diabetes-Induced Aortic Damage Was Associated with the Upregulation of Nrf2 Expression and Function. Oxid. Med. Cell. Longev. 2014, 2014, 123963. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bai, Y.; Zhang, Z.; Xin, Y.; Cai, L. Protection by Sulforaphane from type 1 Diabetes-Induced Testicular Apoptosis Is Associated with the Up-Regulation of Nrf2 Expression and Function. Toxicol. Appl. Pharmacol. 2014, 279, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Si, H.; Babu, P.V.; Pan, D.; Fu, Y.; Brooke, E.A.; Shah, H.; Zhen, W.; Zhu, H.; Liu, D.; et al. Sulforaphane Reduces Vascular Inflammation in Mice and Prevents TNF-alpha-Induced Monocyte Adhesion to Primary Endothelial Cells through Interfering with the NF-kappaB Pathway. J. Nutr. Biochem. 2014, 25, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Liu, D. Genistein, a Soy Phytoestrogen, Upregulates the Expression of Human Endothelial Nitric Oxide Synthase and Lowers Blood Pressure in Spontaneously Hypertensive Rats. J. Nutr. 2008, 138, 297–304. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kraus, D.; Yang, Q.; Kahn, B.B. Lipid Extraction from Mouse Feces. Bio Protoc. 2015, 5, e1375. [Google Scholar] [CrossRef] [PubMed]
- Frisard, M.I.; Wu, Y.; McMillan, R.P.; Voelker, K.A.; Wahlberg, K.A.; Anderson, A.S.; Boutagy, N.; Resendes, K.; Ravussin, E.; Hulver, M.W. Low Levels of Lipopolysaccharide Modulate Mitochondrial Oxygen Consumption in Skeletal Muscle. Metabolism 2015, 64, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Frisard, M.I.; McMillan, R.P.; Marchand, J.; Wahlberg, K.A.; Wu, Y.; Voelker, K.A.; Heilbronn, L.; Haynie, K.; Muoio, B.; Li, L.; et al. Toll-like Receptor 4 Modulates Skeletal Muscle Substrate Metabolism. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E988–E998. [Google Scholar] [CrossRef]
- Hulver, M.W.; Berggren, J.R.; Carper, M.J.; Miyazaki, M.; Ntambi, J.M.; Hoffman, E.P.; Thyfault, J.P.; Stevens, R.; Dohm, G.L.; Houmard, J.A.; et al. Elevated Stearoyl-CoA Desaturase-1 Expression in Skeletal Muscle Contributes to Abnormal Fatty Acid Partitioning in Obese Humans. Cell Metab. 2005, 2, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.T.; Luo, J.; Liu, D. Kaempferol Improves Glucose Uptake in Skeletal Muscle via an AMPK-Dependent Mechanism. Food Sci. Hum. Wellness 2023, 12, 2087–2094. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef]
- Keum, Y.S.; Jeong, W.S.; Kong, A.N.T. Chemoprevention by Isothiocyanates and Their Underlying Molecular Signaling Mechanisms. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2004, 555, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltran, C.E.; Calderon-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective Effect of Sulforaphane against Oxidative Stress: Recent Advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, J.S.; Seo, M.S.; Jung, J.W.; Lee, Y.S.; Kang, K.S. Genistein and Daidzein Repress Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells via Wnt/beta-Catenin Signalling or Lipolysis. Cell Prolif. 2010, 43, 594–605. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent Advances in the Relationship between Obesity, Inflammation, and Insulin Resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Poitout, V. Beta-Cell Glucose Toxicity, Lipotoxicity, and Chronic Oxidative Stress in Type 2 Diabetes. Diabetes 2004, 53 (Suppl. 1), S119–S124. [Google Scholar] [CrossRef] [PubMed]
- Bakker, S.J.; RG, I.J.; Teerlink, T.; Westerhoff, H.V.; Gans, R.O.; Heine, R.J. Cytosolic Triglycerides and Oxidative Stress in Central Obesity: The Missing Link between Excessive Atherosclerosis, Endothelial Dysfunction, and Beta-Cell Failure? Atherosclerosis 2000, 148, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. How Obesity Causes Diabetes: Not a Tall Tale. Science 2005, 307, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-K.; Ren, Z.-N.; Zhu, S.-L.; Wu, Y.-Z.; Wang, G.; Zhang, H.; Chen, W.; He, Z.; Ye, X.-L.; Zhai, Q.-X. Sulforaphane Ameliorates Non-Alcoholic Fatty Liver Disease in Mice by Promoting FGF21/FGFR1 Signaling Pathway. Acta Pharmacol. Sin. 2022, 43, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.M.N.; Jahandideh, F.; Armstrong, E.A.; Bourque, S.L.; Yager, J.Y. Broccoli Sprouts Promote Sex-Dependent Cardiometabolic Health and Longevity in Long-Evans Rats. Int. J. Environ. Res. Public Health 2022, 19, 13468. [Google Scholar] [CrossRef] [PubMed]
- Folbergrová, J.; Ješina, P.; Otáhal, J. Protective Effect of Sulforaphane on Oxidative Stress and Mitochondrial Dysfunction Associated with Status Epilepticus in Immature Rats. Mol. Neurobiol. 2023, 60, 2024–2035. [Google Scholar] [CrossRef]
- Liu, Y.L.; Fu, X.Z.; Chen, Z.Y.; Luo, T.T.; Zhu, C.X.; Ji, Y.T.; Bian, Z. The Protective Effects of Sulforaphane on High-Fat Diet-Induced Obesity in Mice Through Browning of White Fat. Front. Pharmacol. 2021, 12, 665894. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, J.F.; Chen, J.H.; Zhang, Z.W.; Zou, Z.Q.; Han, L.Y.; Hua, Q.H.; Zhao, J.S.; Zhang, X.H.; Shan, Y.J. Sulforaphane Ameliorates Glucose Intolerance in Obese Mice via the Upregulation of the Insulin Signaling Pathway. Food Funct. 2018, 9, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, W.; Cui, S.; Tang, X.; Zhang, Q.; Lu, W.; Jin, Y.; Zhao, J.; Mao, B.; Chen, W. Broccoli Seed Extract Rich in Polysaccharides and Glucoraphanin Ameliorates DSS-Induced Colitis via Intestinal Barrier Protection and Gut Microbiota Modulation in Mice. J. Sci. Food Agric. 2023, 103, 1749–1760. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Alkhalidy, H.; Jia, Z.; Liu, D. Sulforaphane Ameliorates High-Fat-Diet-Induced Metabolic Abnormalities in Young and Middle-Aged Obese Male Mice. Foods 2024, 13, 1055. https://doi.org/10.3390/foods13071055
Luo J, Alkhalidy H, Jia Z, Liu D. Sulforaphane Ameliorates High-Fat-Diet-Induced Metabolic Abnormalities in Young and Middle-Aged Obese Male Mice. Foods. 2024; 13(7):1055. https://doi.org/10.3390/foods13071055
Chicago/Turabian StyleLuo, Jing, Hana Alkhalidy, Zhenquan Jia, and Dongmin Liu. 2024. "Sulforaphane Ameliorates High-Fat-Diet-Induced Metabolic Abnormalities in Young and Middle-Aged Obese Male Mice" Foods 13, no. 7: 1055. https://doi.org/10.3390/foods13071055
APA StyleLuo, J., Alkhalidy, H., Jia, Z., & Liu, D. (2024). Sulforaphane Ameliorates High-Fat-Diet-Induced Metabolic Abnormalities in Young and Middle-Aged Obese Male Mice. Foods, 13(7), 1055. https://doi.org/10.3390/foods13071055