Plant-Based Emulsions as Dairy Cream Alternatives: Comparison of Viscoelastic Properties and Colloidal Stability of Various Model Products
Abstract
:1. Introduction
2. Materials and Methods
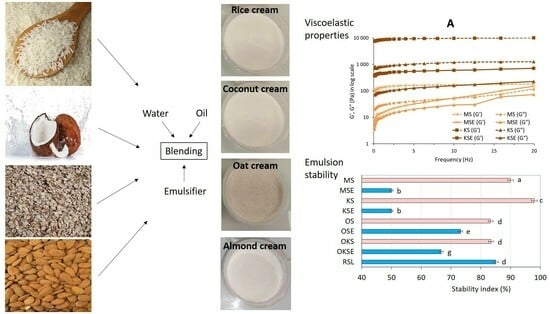
2.1. Plant-Based Creams’ Recipe Ingredients
2.2. Plant-Based Cream Preparation
2.3. Rheological Analysis of Plant-Based Creams
2.4. Texture Analysis of Plant-Based Creams
2.5. Colour Analysis of Plant-Based Creams
2.6. Colloidal Stability of Plant-Based Creams
2.7. Statistical Analysis
3. Results and Discussion
3.1. Rheological Analysis Plant-Based Creams
3.2. Texture Analysis of Plant-Based Creams
3.3. Colour Analysis of Plant-Based Creams
3.4. Colloidal Stability of Plant-Based Creams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClements, D.J. Food Emulsions. Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2005; p. 633. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Liu, C.; Ma, T. Effect of pH-Shifting and Sonication-Assisted Treatment on Properties and Stability of Vegetable Oil-Based Whipped Cream Stabilized by Kidney Bean Protein Aggregates. Food Hydrocoll. 2023, 141, 108736. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, H.; Bai, L.; Rojas, O.J.; McClements, D.J. Development of Food-Grade Pickering Emulsions Stabilized by a Mixture of Cellulose Nanofibrils and Nanochitin. Food Hydrocoll. 2021, 113, 106451. [Google Scholar] [CrossRef]
- Iftikhar, S.A.; Dutta, H. Use of Raw and Physically Modified Rice Starches as Fat Replacer in Whipping Cream. Curr. Res. Nutr. Food Sci. 2020, 8, 122–130. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Zouari, M.; Attia, H.; Besbes, S. Study of Protein/K-Carrageenan Mixture’s Effect on Low-Fat Whipping Cream Formulation. LWT 2021, 147, 111647. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Goli, S.A.H.; Sedaghat Doost, A.; Roman, B.; Dewettinck, K.; Stevens, C.V.; Van der Meeren, P. Plant Based Pickering Stabilization of Emulsions using Soluble Flaxseed Protein and Mucilage Nano-Assemblies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 170–182. [Google Scholar] [CrossRef]
- Semenzato, A.; Costantini, A.; Meloni, M.; Maramaldi, G.; Meneghin, M.; Baratto, G. Formulating O/W Emulsions with Plant-Based Actives: A Stability Challenge for an Effective Product. Cosmetics 2018, 5, 59. [Google Scholar] [CrossRef]
- Derossi, A.; De Pilli, T.; Severini, C. Prediction of Water Activity in Vegetable Creams: Note 2. J. Food Eng. 2007, 79, 1280–1286. [Google Scholar] [CrossRef]
- Verdú, S.; Pérez, A.J.; Barat, J.M.; Grau, R. Laser Backscattering Imaging as a Control Technique for Fluid Foods: Application to Vegetable-Based Creams Processing. J. Food Eng. 2019, 241, 58–66. [Google Scholar] [CrossRef]
- Yiu, C.; Liang, S.; Mukhtar, K.; Kim, W.; Wang, Y.; Selomulya, C. Food Emulsion Gels from Plant-Based Ingredients: Formulation, Processing, and Potential Applications. Gels 2023, 9, 366. [Google Scholar] [CrossRef]
- Morávková, T.; Stern, P. Rheological and Textural Properties of Cosmetic Emulsions. Appl. Rheol. 2011, 21, 35200. [Google Scholar] [CrossRef]
- del Río-Ortuño, Y.; Streitenberger-Jacobi, S.; Bermejo-Fernández, R.; Marín-Iniesta, F. Stability in Plant-Based Creams. An. Vet. Murcia 2022, 36, 1–21. [Google Scholar]
- Nimbkar, S.; Negi, A.; Thirukumaran, R.; Moses, J.A.; Sinija, V.R. Effect of Thermal and Nonthermal Techniques on the Physicochemical Quality of High-fat Coconut Cream. J. Food Process Eng. 2023, 46, e14462. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans Fatty Acids and Lipid Profile: A Serious Risk Factor to Cardiovascular Disease, Cancer and Diabetes. Diabetes Metab. Syndr. 2019, 13, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- da Silva Faresin, L.; Devos, R.J.B.; Reinehr, C.O.; Colla, L.M. Development of Ice Cream with Reduction of Sugar and Fat by the Addition of Inulin, Spirulina Platensis or Phycocyanin. Int. J. Gastron. Food Sci. 2022, 27, 100445. [Google Scholar] [CrossRef]
- Wang, X.; Ma, D.; Liu, Y.; Wang, Y.; Qiu, C.; Wang, Y. Physical Properties of Oleogels Fabricated by the Combination of Diacylglycerols and Monoacylglycerols. J. Am. Oil Chem. Soc. 2022, 99, 1007–1018. [Google Scholar] [CrossRef]
- Lapčíková, B.; Lapčík, L.; Valenta, T.; Majar, P.; Ondroušková, K. Effect of the Rice Flour Particle Size and Variety Type on Water Holding Capacity and Water Diffusivity in Aqueous Dispersions. LWT 2021, 142, 111082. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Reetu; Punia, S.; Dhakane-Lad, J.; Singh, S.; Dhumal, S.; Chandra Pradhan, P.; Bhushan, B.; et al. Functional Characterization of Plant-Based Protein to Determine its Quality for Food Applications. Food Hydrocoll. 2022, 123, 106986. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S.; et al. Cottonseed: A Sustainable Contributor to Global Protein Requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Silva, D.C.d.; Pacheco, M.T.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Oilseed by-Products as Plant-Based Protein Sources: Amino Acid Profile and Digestibility. Future Foods 2021, 3, 100023. [Google Scholar] [CrossRef]
- Shin, J.; Hong, Y.; Lee, K. Development and Physicochemical Properties of Low Saturation Alternative Fat for Whipping Cream. Molecules 2021, 26, 4586. [Google Scholar] [CrossRef]
- Fam, V.W.; Charoenwoodhipong, P.; Sivamani, R.K.; Holt, R.R.; Keen, C.L.; Hackman, R.M. Plant-Based Foods for Skin Health: A Narrative Review. J. Acad. Nutr. Diet. 2022, 122, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Galani, E.; Ly, I.; Laurichesse, E.; Schmitt, V.; Xenakis, A.; Chatzidaki, M.D. Pea and Soy Protein Stabilized Emulsions: Formulation, Structure, and Stability Studies. Colloids Interfaces 2023, 7, 30. [Google Scholar] [CrossRef]
- The European Commission. Commission Regulation (EU) 2019/801 of 17 may 2019 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the use of Mono- and Diglycerides of Fatty Acids (E 471) on Certain Fresh Fruits. Off. J. Eur. Union 2019, 132, 18–20. [Google Scholar]
- Council of the European Union. Council Directive 98/83 about Water Quality Intended for Human Consumption. Off. J. Eur. Communities Legis. 1998, 330, 32–54. [Google Scholar]
- Council of the European Union. Directive 95/2/EC. Food Additives Other than Colours and Sweeteners. Off. J. Eur. Communities 1995, 61, 1–40. [Google Scholar]
- Lapčíková, B.; Valenta, T.; Lapčík, L. Rheological Properties of Food Hydrocolloids Based on Polysaccharides. J. Polym. Mater. 2017, 34, 621–635. [Google Scholar]
- Whitcomb, K. Determining the Linear Viscoelastic Region in Oscillatory Measurements; TA Instruments: New Castle, DE, USA, 2022; Volume RH 107, pp. 1–4. [Google Scholar]
- Bemer, H.L.; Limbaugh, M.; Cramer, E.D.; Harper, W.J.; Maleky, F. Vegetable Organogels Incorporation in Cream Cheese Products. Food Res. Int. 2016, 85, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Pětová, M.; Polášek, Z.; Lapčíková, B.; Lapčík, L.; Buňková, L.; Pospiech, M.; Foltin, P.; Talár, J.; Salek, R.N.; Kůrová, V.; et al. Evaluation of the Viscoelastic Properties of Pork Liver Pâté during Sterilisation Observed In Situ. LWT 2024, 191, 115614. [Google Scholar] [CrossRef]
- Chudy, S.; Gierałtowska, U. Influence of the Background Color on the Cheese Color Parameters. Int. J. Dairy Sci. 2020, 15, 108. [Google Scholar] [CrossRef]
- Rubel, I.A.; Iraporda, C.; Gallo, A.; Manrique, G.D.; Genovese, D.B. Spreadable Ricotta Cheese with Hydrocolloids: Effect on Physicochemical and Rheological Properties. Int. Dairy J. 2019, 94, 7–15. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Li, D.; Guo, L.; Su, X.; Zhang, Y.; Wu, F.; Xu, X. Effect of Pigskin-Originated Gelatin on Properties of Wheat Flour Dough and Bread. Food Hydrocoll. 2019, 94, 183–190. [Google Scholar] [CrossRef]
- Ali, A.H.; Wei, W.; Wang, X. Characterisation of Bovine and Buffalo Anhydrous Milk Fat Fractions along with Infant Formulas Fat: Application of Differential Scanning Calorimetry, Fourier Transform Infrared Spectroscopy, and Colour Attributes. LWT 2020, 129, 109542. [Google Scholar] [CrossRef]
- Wadhwani, R.; McMahon, D.J. Color of Low-Fat Cheese Influences Flavor Perception and Consumer Liking. J. Dairy Sci. 2012, 95, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Nikzade, V.; Tehrani, M.M.; Saadatmand-Tarzjan, M. Optimization of Low-Cholesterol–low-Fat Mayonnaise Formulation: Effect of using Soy Milk and some Stabilizer by a Mixture Design Approach. Food Hydrocoll. 2012, 28, 344–352. [Google Scholar] [CrossRef]
- Espert, M.; Salvador, A.; Sanz, T.; Hernández, M.J. Cellulose Ether Emulsions as Fat Source in Cocoa Creams: Thermorheological Properties (Flow and Viscoelasticity). LWT 2020, 117, 108640. [Google Scholar] [CrossRef]
- Lapčík, L. Gel Form of Matter as a Foundation of the Material-Engineering Elements. Full Professorship Thesis, VUTIUM [Brno University of Technology Publishing House], Brno, Czech Republic, 2002; p. 20. [Google Scholar]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a Phenomenological Definition of the Term ‘gel’. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Burchard, W.; Ross-Murphy, S.B. Physical Networks: Polymers and Gels, 1st ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 1990; p. 418. [Google Scholar]
- Zhang, Y.; Jin, T. Crystal Structure of Cocosin, a Potential Food Allergen from Coconut (Cocos nucifera). J. Allergy Clin. Immunol. 2017, 139, AB261. [Google Scholar] [CrossRef]
- Deffenbaugh, L. Emulsifier-Carbohydrate Interactions. In Food Emulsifiers and Their Applications, 3rd ed.; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 65–100. [Google Scholar] [CrossRef]
- Aisyah, Y.; Irfan; Yunita, D.; Ikhwana, Y. Formulation and Characteristics of Skin Cream with the Addition of Essential Oil Blend. IOP Conf. Ser. Earth Environ. 2024, 1297, 012080. [Google Scholar] [CrossRef]
- Rohmani, S.; Dinda, K.E.; Ainurofiq, A. Formulation and Evaluation of the Cream made from Potassium Azeloyl Diglycinate as an Anti-Aging. J. Phys. Conf. Ser. 2021, 1912, 012041. [Google Scholar] [CrossRef]
- Aydar, A.Y. An Overview of Plant-Based Food Alternatives (PBFAs): Classification, Textural and Sensory Characteristics. In Plant-Based Foods: Ingredients, Technology and Health Aspects, 1st ed.; Aydar, A.Y., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–17. [Google Scholar] [CrossRef]
- Gupta, M.K.; Torrico, D.D.; Ong, L.; Gras, S.L.; Dunshea, F.R.; Cottrell, J.J. Plant and Dairy-Based Yogurts: A Comparison of Consumer Sensory Acceptability Linked to Textural Analysis. Foods 2022, 11, 463. [Google Scholar] [CrossRef]
- Cascone, G.; Crescente, G.; Sorrentino, A.; Volpe, M.G.; Moccia, S. Physicochemical Characterization of a Functional Chestnut Sweet Cream Enriched with Carotenoids and Fiber. LWT 2023, 177, 114583. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.; Chen, W.; Zhong, Q.; Shen, Y.; Zhang, M. Effect of Ultrasound on pH-Shift to Improve Thermal Stability of Coconut Milk by Modifying Physicochemical Properties of Coconut Milk Protein. LWT 2022, 167, 113861. [Google Scholar] [CrossRef]
- Tangsuphoom, N.; Coupland, J.N. Effect of pH and Ionic Strength on the Physicochemical Properties of Coconut Milk Emulsions. J. Food Sci. 2008, 73, E274–E280. [Google Scholar] [CrossRef] [PubMed]
- Nylander, T.; Arnebrant, T.; Cárdenas, M.; Bos, M.; Wilde, P. Protein/Emulsifier Interactions. In Food Emulsifiers and Their Applications, 3rd ed.; Hasenhuettl, G.L., Hartel, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 101–192. [Google Scholar] [CrossRef]
- Yan, G.; Wang, S.; Li, Y.; He, L.; Li, Y.; Zhang, L. Effect of Emulsifier HLB on Aerated Emulsions: Stability, Interfacial Behavior, and Aeration Properties. J. Food Eng. 2023, 351, 111505. [Google Scholar] [CrossRef]
- Zhang, Z.; Goff, H.D. Protein Distribution at Air Interfaces in Dairy Foams and Ice Cream as Affected by Casein Dissociation and Emulsifiers. Int. Dairy J. 2004, 14, 647–657. [Google Scholar] [CrossRef]
- Ariyaprakai, S. Freeze Thaw Stability and Heat Stability of Coconut Oil-in-Water Emulsions and Coconut Milk Emulsions Stabilized by Enzyme-Modified Soy Lecithin. Food Biophys. 2022, 17, 557–567. [Google Scholar] [CrossRef]
- Okuro, P.K.; Gomes, A.; Costa, A.L.R.; Adame, M.A.; Cunha, R.L. Formation and Stability of W/O-High Internal Phase Emulsions (HIPEs) and Derived O/W Emulsions Stabilized by PGPR and Lecithin. Food Res. Int. 2019, 122, 252–262. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Araki, M. Effects of Lecithin Addition in Oil or Water Phase on the Stability of Emulsions made with Whey Proteins. Biosci. Biotechnol. Biochem. 1997, 61, 1791–1795. [Google Scholar] [CrossRef]
- Tan, Y.; McClements, D.J. Plant-Based Colloidal Delivery Systems for Bioactives. Molecules 2021, 26, 6895. [Google Scholar] [CrossRef]
- Reiner, J.; Schüler, C.; Gaukel, V.; Karbstein, H.P. Effects of Cooling Rate and Emulsifier Combination on the Colloidal Stability of Crystalline Dispersions Stabilized by Phospholipids and β-Lactoglobulin. Colloids Interfaces 2023, 7, 45. [Google Scholar] [CrossRef]





| Sample (Abbreviation) | Ingredient Ratio (vol. %) a | Water Content (mL) | Oil Content (mL) | o/w b | Guar Gum (g) | Fine Rice Flour (g) | Salt (g) |
|---|---|---|---|---|---|---|---|
| Creams without emulsifier | |||||||
| Almond cream (MS) | 38.2 | 250 | 25 | 0.10 | 0.25 | - c | 1.0 |
| Coconut cream (KS) | 53.0 | 250 | 25 | 0.10 | 0.50 | 50 | 1.0 |
| Oat cream (OS) | 46.0 | 250 | 25 | 0.10 | 0.50 | - | 1.0 |
| Oat–coconut cream (OKS) | 49.0 | 250 | 25 | 0.10 | 0.50 | 25 | 1.0 |
| Creams with mono- and diglycerides of fatty acids (0.75 g/100 g) | |||||||
| Almond cream (MSE) | 38.2 | 250 | 25 | 0.10 | 0.50 | - | 1.0 |
| Coconut cream (KSE) | 53.0 | 250 | 25 | 0.10 | 0.50 | 50 | 1.0 |
| Oat cream (OSE) | 46.0 | 250 | 25 | 0.10 | 0.50 | - | 1.0 |
| Oat–coconut cream (OKSE) | 49.0 | 250 | 25 | 0.10 | 0.50 | 25 | 1.0 |
| Cream with lecithin (0.40 g/100 g) | |||||||
| Rice cream (RSL) | 11.1 | 700 | 100 | 0.14 | 0.70 | 25 | 2.0 |
| Sample | Herschel–Bulkley Model | Ostwald–de Waele Model | |||||
|---|---|---|---|---|---|---|---|
| τ0 (Pa) | k (Pa·sn) | n | R2 | k (Pa·sn) | n | R2 | |
| MS | 0.91 ± 0.04 ad | 0.26 ± 0.02 a | 0.75 ± 0.03 a | 0.9948 | 1.30 ± 0.05 a | 0.34 ± 0.01 a | 0.9521 |
| MSE | 0.29 ± 0.01 a | 0.57 ± 0.03 a | 0.59 ± 0.02 b | 0.9990 | 0.98 ± 0.03 a | 0.43 ± 0.02 b | 0.9802 |
| KS | 34.65 ± 1.68 b | 1.71 ± 0.07 a | 0.72 ± 0.02 ac | 0.9553 | 2.08 ± 0.08 a | 0.69 ± 0.03 c | 0.9538 |
| KSE | 0.00 ± 0.00 a | 47.39 ± 1.56 b | 0.24 ± 0.01 d | 0.9991 | 18.25 ± 0.57 b | 0.45 ± 0.01 b | 0.9858 |
| OS | 0.00 ± 0.00 a | 204.90 ± 6.04 c | 0.20 ± 0.01 d | 0.9742 | 89.92 ± 2.14 c | 0.35 ± 0.01 a | 0.9915 |
| OSE | 6.77 ± 0.29 c | 2.04 ± 0.08 a | 0.67 ± 0.03 c | 0.9992 | 9.95 ± 0.40 d | 0.28 ± 0.01 d | 0.9552 |
| OKS | 2.26 ± 0.11 d | 15.41 ± 0.59 d | 0.51 ± 0.02 e | 1.0 | 18.20 ± 0.61 b | 0.47 ± 0.02 be | 0.9995 |
| OKSE | 0.00 ± 0.00 a | 11.37 ± 0.41 d | 0.45 ± 0.01 e | 1.0 | 9.17 ± 0.34 d | 0.51 ± 0.02 ef | 0.9983 |
| RSL | 5.64 ± 0.25 c | 10.21 ± 0.23 d | 0.66 ± 0.03 bc | 0.9974 | 16.34 ± 0.55 b | 0.54 ± 0.02 f | 0.9800 |
| Sample | L* | a* | b* | h* (°) | C* |
|---|---|---|---|---|---|
| MS | 86.40 ± 0.02 ab | 1.56 ± 0.00 a | 12.96 ± 0.03 ab | 83.13 ± 0.01 ab | 13.05 ± 0.02 ac |
| MSE | 86.10 ± 0.01 ab | 1.54 ± 0.00 a | 12.71 ± 0.00 ab | 83.09 ± 0.00 ab | 12.80 ± 0.00 ac |
| KS | 84.47 ± 0.14 ac | −0.97 ± 0.01 b | 5.94 ± 0.03 c | 80.76 ± 0.10 b | 6.01 ± 0.03 b |
| KSE | 88.27 ± 0.11 b | −0.85 ± 0.00 b | 6.11 ± 0.00 c | 82.08 ± 0.00 b | 6.17 ± 0.00 b |
| OS | 81.56 ± 0.01 de | 1.26 ± 0.00 ac | 14.35 ± 0.00 a | 84.98 ± 0.00 c | 14.40 ± 0.00 a |
| OSE | 82.84 ± 0.02 cd | 0.90 ± 0.01 cd | 12.99 ± 0.00 ab | 86.06 ± 0.02 cd | 13.02 ± 0.01 ac |
| OKS | 84.37 ± 0.00 c | 0.62 ± 0.01 d | 11.89 ± 0.00 b | 87.04 ± 0.02 d | 11.90 ± 0.01 c |
| OKSE | 82.59 ± 0.21 cd | 0.55 ± 0.02 d | 10.10 ± 0.09 b | 86.86 ± 0.11 d | 10.12 ± 0.09 d |
| RSL | 80.12 ± 0.00 e | −1.94 ± 0.01 e | 4.15 ± 0.01 c | 65.00 ± 0.11 e | 4.58 ± 0.01 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapčíková, B.; Lapčík, L.; Valenta, T.; Chvatíková, M. Plant-Based Emulsions as Dairy Cream Alternatives: Comparison of Viscoelastic Properties and Colloidal Stability of Various Model Products. Foods 2024, 13, 1225. https://doi.org/10.3390/foods13081225
Lapčíková B, Lapčík L, Valenta T, Chvatíková M. Plant-Based Emulsions as Dairy Cream Alternatives: Comparison of Viscoelastic Properties and Colloidal Stability of Various Model Products. Foods. 2024; 13(8):1225. https://doi.org/10.3390/foods13081225
Chicago/Turabian StyleLapčíková, Barbora, Lubomír Lapčík, Tomáš Valenta, and Marie Chvatíková. 2024. "Plant-Based Emulsions as Dairy Cream Alternatives: Comparison of Viscoelastic Properties and Colloidal Stability of Various Model Products" Foods 13, no. 8: 1225. https://doi.org/10.3390/foods13081225
APA StyleLapčíková, B., Lapčík, L., Valenta, T., & Chvatíková, M. (2024). Plant-Based Emulsions as Dairy Cream Alternatives: Comparison of Viscoelastic Properties and Colloidal Stability of Various Model Products. Foods, 13(8), 1225. https://doi.org/10.3390/foods13081225