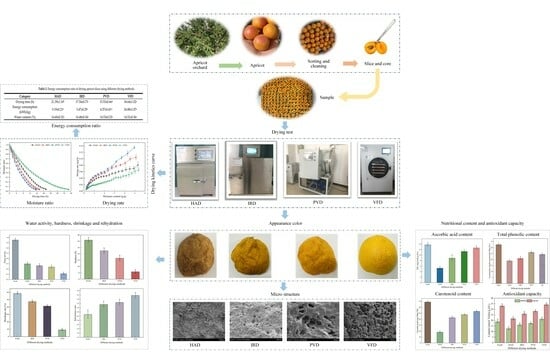
Effects of Different Drying Methods on Drying Characteristics, Microstructure, Quality, and Energy Consumption of Apricot Slices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drying Method
2.2.1. Hot Air Drying
2.2.2. Infrared Drying
2.2.3. Vacuum Freeze-Drying
2.2.4. Pulsed Vacuum Drying
2.3. Drying Kinetics
2.4. Energy Consumption Ratio
2.5. Color
2.6. Water Activity
2.7. Hardness
2.8. Shrinkage
2.9. Rehydration Ratio
2.10. Microstructure
2.11. Nutritional Characteristics
2.11.1. Ascorbic Acid Content
2.11.2. Total Phenol Content
2.11.3. Total Carotenoid Content
2.12. Determination of Antioxidant Activity
2.12.1. DPPH Determination
2.12.2. FRAP Determination
2.13. Data Processing
3. Results and Discussion
3.1. Effects of Different Drying Methods on the Drying Time and Energy Consumption Ratio of Apricot Slices
3.2. Effects of Different Drying Methods on the Color of Apricot Slices
3.3. Effects of Different Drying Methods on the Water Activity of Apricot Slices
3.4. Effects of Different Drying Methods on the Hardness and Shrinkage of Apricot Slices
3.5. Effects of Different Drying Methods on the Rehydration of Apricot Slices
3.6. Effect of Different Drying Methods on the Microstructure of Apricot Slices
3.7. Effects of Different Drying Methods on Ascorbic Acid and Total Phenols in Apricot Slices
3.8. Effects of Different Drying Methods on Carotenoids in Apricot Slices
3.9. Effects of Different Drying Methods on the Antioxidant Activity of Apricot Slices
3.10. Energy Consumption Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, O.E.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization and the effect of maturity at harvest on the phenolic and carotenoid content of northeast USA apricot (Prunus armeniaca) Varieties. J. Agric. Food Chem. 2013, 61, 12700–12710. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Wu, Q.Y.; Li, Y.N.; Wang, S.F. Effects of vacuum impregnation with calcium lactate and pectin methylesterase on quality attributes and chelate-soluble pectin morphology of fresh-cut papayas. Food Bioprocess Technol. 2017, 10, 901–913. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, J.; Pu, X.; Shi, X.; Cheng, W.; Wang, B. Volatile Compounds Analysis and Biomarkers Identification of Four Native Apricot (Prunus armeniaca L.). Cultiv. Grown Xinjiang Reg. China Foods 2022, 11, 2297. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhou, L.Y.; Song, H.B.; Yi, J.Y.; Wu, B.; Li, Y.R.; Zhang, L.; Che, F.B.; Wang, Z.D.; Gao, M.X.; et al. Electron beam irradiation of sun-dried apricots for quality maintenance. Radiat. Phys. Chem. 2014, 97, 126–133. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Xiao, Y.D.; Lagnika, C.; Song, J.F.; Li, D.J.; Liu, C.Q.; Jiang, N.; Zhang, M.; Duan, X. A comparative study of drying methods on physical characteristics, nutritional properties and antioxidant capacity of broccoli. Dry. Technol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef]
- Kayran, S.; Doymaz, I. Infrared Drying and Effective Moisture Diffusivity of Apricot Halves: Influence of Pretreatment and Infrared Power. J. Food Process. Preserv. 2016, 17, 12827. [Google Scholar] [CrossRef]
- Ihns, R.; Diamante, L.M.; Savage, G.P.; Vanhanen, L. Effect of temperature on the drying characteristics, colour, antioxidant and beta-carotene contents of two apricot varieties. Int. J. Food Sci. Technol. 2011, 46, 275–283. [Google Scholar] [CrossRef]
- Karabulut, I.; Topcu, A.; Duran, A.; Turan, S.; Ozturk, B. Effect of hot air drying and sun drying on color values and β-carotene content of apricot (Prunus armenica L.). LWT-Food Sci. Technol. 2007, 40, 753–758. [Google Scholar] [CrossRef]
- Zartha Sossa, J.W.; Orozco, G.L.; García Murillo, L.M.; Peña Osorio, M.; Sánchez Suarez, N. Infrared drying trends applied to fruit. Front. Sustain. Food Syst. 2021, 5, 650590. [Google Scholar] [CrossRef]
- Karatas, F.; Kamışlı, F. Variations of vitamins (A, C and E) and MDA in apricots dried in IR and microwave. J. Food Eng. 2007, 78, 662–668. [Google Scholar] [CrossRef]
- Jin, L.W.; Ren, G.Y.; Duan, X.; Zhang, Y.M. Effect of ultrasonic synergy on the dehydration and quality of vacuum freeze-dried apricots. Food Ferment. Ind. 2020, 46, 133–139. [Google Scholar] [CrossRef]
- Jiang, D.L.; Xiao, H.W.; Zheng, Z.A. Effects of different drying methods on drying characteristics, microstructure, quality, and energy consumption of Panax Notoginseng roots (Araliaceae). Dry. Technol. 2022, 40, 1247–1261. [Google Scholar] [CrossRef]
- Wang, A.N.; Wang, Y.; Kan, H.; Hao, J.B.; Hu, Q.; Lu, B.; Liu, Y. Comparison of different drying techniques for Zanthoxylum bungeanum leaves: Changes in color, microstructure, antioxidant capacities, and volatile components. LWT-Food Sci. Technol. 2023, 188, 115469. [Google Scholar] [CrossRef]
- Link, J.V.; Tribuzi, G.; Laurindo, J.B. Conductive multi-flash drying of mango slices: Vacuum pulse conditions on drying rate and product properties. J. Food Process. Preserv. 2017, 42, e13440. [Google Scholar] [CrossRef]
- Bai, J.W. Drying Kinetics and Anti-Browning Mechanism of Thompson Seedless Grapes. Doctoral Dissertation, China Agricultural University, Beijing, China, 2014. [Google Scholar]
- Yang, K.W.; Wang, D.; Vidyarthi, S.K.; Li, S.B.; Liu, Z.L.; Wang, H.; Chen, X.J.; Xiao, H.W. Pulsed Vacuum Drying of Persimmon Slices: Drying Kinetics, Physicochemical Properties, Microstructure and Antioxidant Capacity. Plants 2022, 11, 2500. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.H.; Wu, Z.F.; Wang, X.C.; Wan, N.; Yang, M. Dehydration of wolfberry fruit using pulsed vacuum drying combined with carboxymethyl cellulose coating pretreatment. LWT-Food Sci. Technol. 2020, 134, 110159. [Google Scholar] [CrossRef]
- Zheng, Z.A.; Wang, S.Y.; Wang, H.; Xiao, H.M.; Liu, Z.L.; Pan, Y.H.; Gao, L. Comparative Study on the Influence of Various Drying Techniques on Drying Characteristics and Physicochemical Quality of Garlic Slices. Foods 2023, 12, 1314. [Google Scholar] [CrossRef]
- Chu, Q.Q.; Li, L.; Duan, X.; Zhao, M.Y.; Wang, Z.K.; Wang, Z.; Ren, X.; Li, C.Y.; Ren, G.Y. Effect mechanism of different drying methods on the quality and browning for daylily. LWT-Food Sci. Technol. 2023, 182, 114862. [Google Scholar] [CrossRef]
- Yang, R.L.; Li, Q.; Hu, Q.P. Physicochemical properties, microstructures, nutritional components, and free amino acids of Pleurotus eryngii as affected by different drying methods. Sci. Rep. 2020, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Mujumdar, A.S.; Lim, R.X. Comparison of drying characteristic and uniformity of banana cubes dried by pulse-spouted microwave vacuum drying, freeze drying and microwave freeze drying. J. Sci. Food Agric. 2014, 94, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- An, N.N.; Zhao, S.Y.; Li, D.; Wang, Y.; Wang, L.J. Flow Rolling Dry-Blanching Pretreatment Improves the Drying Quality of Acanthopanax sessiliflorus by Increasing the Drying Rate and Inactivating Enzymes. Foods 2022, 11, 3186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Li, Z.L.; Bi, J.F.; Zhou, L.Y.; Yi, J.Y.; Wu, X.Y. Effect of hybrid drying methods on physicochemical, nutritional and antioxidant properties of dried black mulberry. LWT-Food Sci. Technol. 2017, 80, 178–184. [Google Scholar] [CrossRef]
- Juhnevica-Radenkova, K.; Krasnova, I.; Seglina, D.; Kaufmane, E.; Gravite, I.; Valdovska, A.; Radenkovs, V. Biochemical Profile and Antioxidant Activity of Dried Fruit Produced from Apricot Cultivars Grown in Latvia. Horticulturae 2024, 10, 205. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Z.H.; Jiao, Y.F.; Huang, J.S.; Yu, Z.; Zhang, D.; Chen, Y.Q.; Ni, D.J. Hot-Air Drying Significantly Improves the Quality and Functional Activity of Orange Black Tea Compared with Traditional Sunlight Drying. Foods 2023, 12, 1913. [Google Scholar] [CrossRef] [PubMed]
- Man, X.L.; Li, L.; Fan, X.W.; Zhang, H.; Lan, H.P.; Tang, Y.R.; Zhang, Y.C. Drying Kinetics and Mass Transfer Characteristics of Walnut under Hot Air Drying. Agriculture 2024, 14, 182. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lv, W.Q.; Lin, R.H.; Li, D.; Wang, L.J. Drying characteristics and bioactivity evolution of Platycodon grandiflorum as affected by different microwave combined drying methods. Int. J. Food Eng. 2021, 17, 395–401. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Xiao, Y.D.; Lagnika, C.; Li, D.J.; Liu, C.Q.; Jiang, N.; Zhang, M. A comparative evaluation of nutritional properties, antioxidant capacity and physical characteristics of cabbage (Brassica oleracea var. Capitate var. L.) subjected to different drying methods. Food Chem. 2020, 309, 124935. [Google Scholar] [CrossRef]
- Brütsch, L.; Rugiero, S.; Serrano, S.S.; Städeli, C.; Windhab, E.J.; Fischer, P.; Kuster, S. Targeted inhibition of enzymatic browning in wheat pastry dough. J. Agric. Food Chem. 2018, 66, 12353–12360. [Google Scholar] [CrossRef] [PubMed]
- Kožar, I.; Rukavina, T. The effect of material density on load rate sensitivity in nonlinear viscoelastic material models. Arch. Appl. Mech. 2019, 89, 873–883. [Google Scholar] [CrossRef]
- Wang, H.C.; Zhang, M.; Mujumdar, A.S. Comparison of three new drying methods for drying characteristics and quality of shiitake mushroom (Lentinus edodes). Dry. Technol. 2014, 32, 1791–1802. [Google Scholar] [CrossRef]
- Baysal, T.; Icier, F.; Ersus, S.; Yıldız, H. Effects of microwave and infrared drying on the quality of carrot and garlic. Eur. Food Res. Technol. 2003, 218, 68–73. [Google Scholar] [CrossRef]
- Šumić, Z.; Tepić, A.; Vidović, S.; Jokić, S.; Malbaša, R. Optimization of frozen sour cherries vacuum drying process. Food Chem. 2013, 136, 55–63. [Google Scholar] [CrossRef] [PubMed]
- González, C.M.; Llorca, E.; Quiles, A.; Hernando, I.; Moraga, G. Water sorption and glass transition in freeze-dried persimmon slices. Effect on physical properties and bioactive compounds. LWT-Food Sci. Technol. 2020, 130, 109633. [Google Scholar] [CrossRef]
- Kręcisz, M.; Stępień, B.; Pasławska, M.; Popłoński, J.; Dulak, K. Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods. Molecules 2021, 26, 4597. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.L.; Xiao, H.W.; Zielinska, M.; Zhu, G.F.; Bai, T.Y.; Zheng, Z.A. Effect of pulsed vacuum drying on drying kinetics and quality of roots of Panax notoginseng (Burk.) F. H. Chen (Araliaceae). Dry. Technol. 2021, 39, 2234–2251. [Google Scholar] [CrossRef]
- Zhao, S.Y.; An, N.N.; Zhang, K.Y.; Li, D.; Wang, L.J.; Wang, Y. Evaluation of drying kinetics, physical properties, bioactive compounds, antioxidant activity and microstructure of Acanthopanax sessiliflorus fruits dried by microwave-assisted hot air drying method. J. Food Eng. 2023, 357, 111642. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, M.; Mujumdar, A.S.; Mothibe, K.J. Quality Changes of Dehydrated Restructured Fish Product from Silver Carp (Hypophthalmichthys molitrix) as Affected by Drying Methods. Food Bioprocess Technol. 2013, 6, 1664–1680. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Jiang, L.; Xu, Z.C.; Zhang, X.J.; Fan, Y.B.; Adnouni, M.; Zhang, C.B. Optimization of a variable-temperature heat pump drying process of shiitake mushrooms using response surface methodology. Renew. Energy 2022, 198, 1267–1278. [Google Scholar] [CrossRef]
- Wang, H.X.; Xie, H.X.; Chen, S.J.; Fu, Q.Q.; Wang, R.R.; Zhang, W.; Hu, Z.C. Effect of different drying methods on drying characteristics and qualities of lemon slices. Trans. Chin. Soc. Agric. Eng. 2017, 33, 292–299. [Google Scholar] [CrossRef]
- Xie, L.; Mujumdar, A.S.; Fang, X.M.; Wang, J.; Dai, J.W.; Du, Z.L.; Xiao, H.W.; Liu, Y.H.; Gao, Z.J. Far-Infrared Radiation Heating Assisted Pulsed Vacuum Drying (FIR-PVD) of Wolfberry (Lycium barbarum L.): Effects on Drying Kinetics and Quality Attributes. Food Bioprod. Process 2017, 102, 320–331. [Google Scholar] [CrossRef]
- Kayacan, S.; Karasu, S.; Akman, P.K.; Goktas, H.; Doymaz, I.; Sagdic, O. Effect of different drying methods on total bioactive compounds, phenolic profile, in vitro bioaccessibility of phenolic and HMF formation of persimmon. LWT-Food Sci. Technol. 2020, 118, 108830. [Google Scholar] [CrossRef]
- Wang, L.Q.; Hu, Q.H.; Pei, F.; Mugambi, M.A.; Yang, W.J. Influence of different storage conditions on physical and sensory properties of freeze-dried Agaricus bisporus slices. LWT-Food Sci. Technol. 2018, 97, 164–171. [Google Scholar] [CrossRef]
- Li, M.J.; Zhang, Y.Y.; You, X.R.; Wang, Y.; Zhou, K.; Wei, P.; Wei, L.Y. Effects of different drying technologies on the quality characteristics and microstructure of walnut meal protein powder. Food Sci. 2021, 42, 92–98. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Tomás-Barberán, F.A.; Gil, M.I. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef] [PubMed]
- Bourvellec, C.L.; Gouble, B.; Bureau, S.; Reling, P.; Bott, R.; Ribas-Agusti, A.; Audergon, J.; Renard, C.M. Impact of canning and storage on apricot carotenoids and polyphenols. Food Chem. 2018, 240, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Garza, I.P.; Ramos-Parra, P.A.; Hernández-Brenes, C.; Jacobo-Velázquez, D.A. Effects of postharvest ripening on the nutraceutical and physicochemical properties of mango (Mangifera indica L. cv Keitt). Postharvest Biol. Technol. 2015, 103, 45–54. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Feng, Y.B.; ElGasim, A.Y.A.; Sun, Y.H.; Ma, H.L.; Zhou, C.S. Vacuum pulsation drying of okra (Abelmoschus esculentus L. Moench): Better retention of the quality characteristics by flat sweep frequency and pulsed ultrasound pretreatment. Food Chem. 2020, 326, 127026. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Juhaimi, F.A.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. LWT-Food Sci. Technol. 2020, 126, 109354. [Google Scholar] [CrossRef]
- Dziedzic, E.; Baszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopeć, A. Effect of modified (MAP) and controlled atmosphere (CA) storage on the quality and bioactive compounds of blue honeysuckle fruits (Lonicera caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.L.; Vidyarthi, S.K.; Wang, Q.H.; Gao, L.; Li, B.R.; Wei, Q.; Liu, Y.H.; Xiao, H.W. Effects of different drying methods on drying kinetics, physicochemical properties, microstructure, and energy consumption of potato (Solanum tuberosum L.) cubes. Dry. Technol. 2021, 39, 418–431. [Google Scholar] [CrossRef]














| Category | Fresh Sample | HAD | IRD | PVD | VFD |
|---|---|---|---|---|---|
 |  |  |  |  | |
| L | 65.60 ± 0.98 b | 43.56 ± 2.16 e | 50.82 ± 0.95 d | 54.27 ± 0.44 c | 70.29 ± 0.84 a |
| a | 17.54 ± 1.14 c | 19.45 ± 1.18 c | 28.67 ± 0.89 a | 25.47 ± 0.96 b | 24.02 ± 1.25 b |
| b | 47.19 ± 1.49 c | 33.23 ± 3.19 d | 46.49 ± 1.24 c | 52.26 ± 0.86 b | 58.07 ± 1.54 a |
| - | 26.45 ± 1.36 a | 18.72 ± 0.41 b | 14.89 ± 0.50 c | 13.64 ± 0.55 c | |
| C | 50.35 ± 1.48 d | 38.52 ± 3.06 e | 54.62 ± 1.49 c | 58.15 ± 0.39 b | 62.84 ± 1.87 a |
| Browning degree (AU/g) | - | 1.54 ± 0.14 a | 0.89 ± 0.67 b | 0.50 ± 0.08 c | 0.35 ± 0.04 c |
| Category | HAD | IRD | PVD | VFD |
|---|---|---|---|---|
| Drying time (h) | 21.39 ± 1.10 b | 17.54 ± 0.73 c | 15.53 ± 0.66 d | 34.64 ± 1.02 a |
| Energy consumption (kWh/kg) | 5.19 ± 0.21 b | 3.47 ± 0.29 c | 4.57 ± 0.41 bc | 26.88 ± 1.07 a |
| Water content (%) | 14.60 ± 0.32 a | 14.48 ± 0.34 a | 14.53 ± 0.23 a | 14.51 ± 0.36 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Yi, X.; Xiao, H.; Wang, X.; Liu, L.; Tang, Z.; Hu, C.; Li, X. Effects of Different Drying Methods on Drying Characteristics, Microstructure, Quality, and Energy Consumption of Apricot Slices. Foods 2024, 13, 1295. https://doi.org/10.3390/foods13091295
Yang Q, Yi X, Xiao H, Wang X, Liu L, Tang Z, Hu C, Li X. Effects of Different Drying Methods on Drying Characteristics, Microstructure, Quality, and Energy Consumption of Apricot Slices. Foods. 2024; 13(9):1295. https://doi.org/10.3390/foods13091295
Chicago/Turabian StyleYang, Qiaonan, Xiaokang Yi, Hongwei Xiao, Xufeng Wang, Lin Liu, Ziya Tang, Can Hu, and Xibing Li. 2024. "Effects of Different Drying Methods on Drying Characteristics, Microstructure, Quality, and Energy Consumption of Apricot Slices" Foods 13, no. 9: 1295. https://doi.org/10.3390/foods13091295
APA StyleYang, Q., Yi, X., Xiao, H., Wang, X., Liu, L., Tang, Z., Hu, C., & Li, X. (2024). Effects of Different Drying Methods on Drying Characteristics, Microstructure, Quality, and Energy Consumption of Apricot Slices. Foods, 13(9), 1295. https://doi.org/10.3390/foods13091295