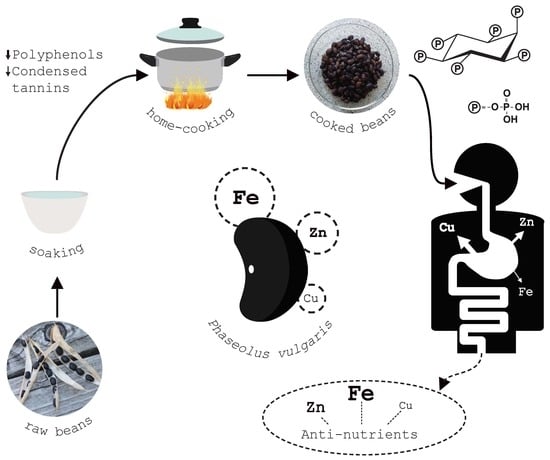
Effect of Traditional Household Processes on Iron, Zinc and Copper Bioaccessibility in Black Bean (Phaseolus vulgaris L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Household Treatments
2.3. Myo-Inositol Phosphates
2.4. Total Polyphenols
2.5. Condensed Tannins
2.6. Minerals
2.7. Iron, Zinc and Copper Bioaccessibility
2.8. Statistical Analysis
3. Results and Discussion
3.1. Mineral Contents of Raw and Cooked Beans
3.2. Anti-Nutrients
3.3. Bioaccessibility of Iron, Zinc and Copper
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Limitations
References
- World Health Organization (WHO). Nutrition Topics. Micronutrient Deficiencies. Available online: http://www.who.int/nutrition/topics/micronutrients/en/ (accessed on 23 May 2018).
- World Health Organization (WHO). The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Available online: http://www.who.int/whr/2002/en/whr02_en.pdf (accessed on 23 May 2018).
- World Health Organization (WHO). World Health Statistics. Available online: www.who.int/whosis/whostat/2011/en/ (accessed on 23 May 2018).
- World Health Organization (WHO). Global Health Risks, Part 2, Results. Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_part2.pdf (accessed on 30 May 2018).
- Hambidge, K.M. Mild zinc deficiency in human subjects. In Zinc in Human Biology; Mills, C.F., Ed.; Springer: New York, NY, USA, 1989; pp. 281–296. [Google Scholar]
- Lazarchick, J. Update on anemia and neutropenia in copper deficiency. Curr. Opin. Hematol. 2012, 19, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.T.; Ming, H.; Megharaj, M.; Naidu, R. Heavy metal (Cu, Zn, Cd and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. J. Hazard Mater. 2009, 171, 1150–1158. [Google Scholar] [CrossRef]
- Lima, A.C.S.; Soares, D.J.; Silva, L.M.R.; Figueiredo, R.W.; Sousa, P.H.M.; Menezes, E.A. In Vitro bioaccessibility of copper, iron, zinc and antioxidant compounds of whole cashew apple juice and cashew apple fibre (Anacardium occidentale L.) following simulated gastro-intestinal digestion. Food Chem. 2014, 161, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.S. Bioavailability of minerals in legumes. Br. J. Nutr. 2002, 88, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, S.M.F. Biodisponibilidade de minerais. Rev. Nutr. 1997, 88, 87–98. [Google Scholar] [CrossRef]
- Scheers, N. Regulatory effects of Cu, Zn, and Ca on Fe absorption: The intricate play between nutrient transporters. Nutrients 2013, 5, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Pizarro, F.; Ruz, M.; De Romaña, D.L. Acute inhibition of iron bioavailability by zinc: Studies in humans. Biometals 2012, 25, 657–664. [Google Scholar] [CrossRef]
- Raes, K.; Knockaert, D.; Struijs, K.; Van Camp, J. Role of processing on bioaccessibility of minerals: Influence of localization of minerals and anti-nutritional factors in the plant. Trends Food Sci. Technol. 2014, 37, 32–41. [Google Scholar] [CrossRef]
- Hoppler, M.; Zeder, C.; Walczyk, T. Quantification of ferritin-bound iron in plant samples by isotope tagging and species-specific isotope dilution mass spectrometry. Anal. Chem. 2009, 81, 7368–7372. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). FAO/INFOODS Global Food Composition Database for Pulses Version 1.0-uPulses 1.0. Available online: http://www.fao.org/infoods/infoods/tables-and-databases/faoinfoods-databases/en/ (accessed on 30 April 2018).
- Akibode, S.; Maredia, M.K. Global and Regional Trends in Production, Trade and Consumption of Food Legume Crops; Michigan State University: East Lansing, MI, USA, 2011. [Google Scholar]
- Lovato, F.; Kowaleski, J.; Silva, S.Z.; Heldt, L.F.S. Composição centesimal e conteúdo mineral de diferentes cultivares de feijão biorfortificado (Phaseolus vulgaris L.). Brazilian J. Food Technol. 2018, 21, 1–6. [Google Scholar] [CrossRef]
- Suliburska, J.; Krejpcio, Z. Evaluation of the content and bioaccessibility of iron, zinc, calcium and magnesium from groats, rice, leguminous grains and nuts. J. Food Sci. Technol. 2014, 51, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.J.; Carvalho, L.M.; Dellamora-Ortiz, G.M.; Cardoso, F.S.; Carvalho, J.L. Effect of different home-cooking methods on the bioaccessibility of zinc and iron in conventionally bred cowpea (Vigna unguiculata L. Walp) consumed in Brazil. Food Nutr. Res. 2016, 60, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Boy, E.; Wirth, J.P.; Hurrell, R.F. The potential of the common bean (Phaseolus vulgaris) as a vehicle for iron biofortification. Nutrients 2015, 7, 1144–1173. [Google Scholar] [CrossRef] [PubMed]
- Companhia Nacional de Abastecimento (Conab). A Cultura do Feijão, 2018; Conab: Brasília, Brazil, 2018; ISBN 978-85-62223-12-9. Available online: http://www.conab.gov.br (accessed on 19 July 2018).
- Institute Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222312/ (accessed on 8 March 2018).
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr. Bull. 2010, 31, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Cuadrado, C.; Burbano, C.; Allaf, K.; Haddad, J.; Gelencsér, E.; Takács, K.; Guillamón, E.; Muzquiz, M. Effect of instant controlled pressure drop on the oligosaccharides, inositol phosphates, trypsin inhibitors and lectins contents of different legumes. Food Chem. 2012, 131, 862–868. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Nishida, W.; Da Costa Proença, R.P. Influence of soaking on the nutritional quality of common beans (Phaseolus vulgaris L.) cooked with or without the soaking water: A review. Int. J. Food Sci. Technol. 2010, 45, 2209–2218. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, In Vitro iron and calcium bioavailability and In Vitro starch and protein digestibility of some legume seeds. LWT-Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and Phytic Acid Contribute to the Low Iron Bioavailability from Common Beans in Young Women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Agric. Food Chem. 2017, 57, 4754–4764. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Jiménez, A.K.; Reynoso-Camacho, R.; Tejero, M.E.; León-Galván, F.; Loarca-Piña, G. Potential role of bioactive compounds of Phaseolus vulgaris L. on lipid-lowering mechanisms. Food Res. Int. 2015, 76, 92–104. [Google Scholar] [CrossRef]
- Hart, J.J.; Tako, E.; Kochian, L.V.; Glahn, R.P. Identification of black bean (Phaseolus vulgaris L.) polyphenols that inhibit and promote iron uptake by Caco-2 cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.D.T.; Guy, A.C. A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int. J. Gastronomy Food Sci. 2016, 3, 2–11. [Google Scholar] [CrossRef]
- Feitosa, S.; Korn, M.G.; Pinelli, M.; Oliveira, T.; Boffo, E.; Greiner, R.; Almeida, D.T. Content of Minerals and Antinutritional Factors in Akara (Fried Cowpea Food). Int. J. Food Process Technol. 2015, 2, 42–50. [Google Scholar] [CrossRef]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of In Vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D and E. Fron. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef] [PubMed]
- Perina, E.F.; Carvalho, C.R.L.; Chiorato, A.F.; Lopes, R.L.T.; Gonçalves, J.G.R.; Carbonell, S.A.M. Technological quality of common bean grains obtained in different growing seasons. Bragantia 2014, 73, 14–22. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Phytate in Foods, Anion-Exchange Method, No. 986.11, 15th ed.; Official Methods of Analysis AOAC: Arlington, VA, USA, 1990; pp. 800–801. [Google Scholar]
- Menezes-Blackburn, D.; Gabler, S.; Greiner, R. Performance of Seven Commercial Phytases in an In Vitro Simulation of Poultry Digestive Tract. J. Agric. Food Chem. 2015, 63, 6142–6149. [Google Scholar] [CrossRef] [PubMed]
- King, H.G.; Health, G.W. The chemical analysis of small samples of leaf material and the relationship between disappearance and composition of leaves. Pedobiologia 1967, 7, 192–197. [Google Scholar]
- Broadhurst, R.B.; Jones, W.J. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–792. [Google Scholar] [CrossRef]
- Rebellato, A.P.; Bussi, J.; Silva, J.G.S.; Greiner, R.; Steel, C.J.; Pallone, J.A.L. Effect of different iron compounds on rheological and technological parameters as well as bioaccessibility of minerals in whole wheat bread. Food Res. Int. 2017, 94, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Katzenberg, M.A.; Saunders, S.R.; Abonyi, S. Bone Chemistry, Food and History: A Case Study from 19th Century Upper Canada. In Biogeochemical Approaches to Paleodietary Analysis; Ambrose, S.H., Katzenberg, M.A., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2002. [Google Scholar]
- Quintaes, K.D.; Amaya-Farfan, J.; Tomazini, F.M.; Morgano, M.A.; Mantovani, D.M. Migração de minerais de panelas brasileiras de aço inoxidável, ferro fundido e pedra-sabão (esteatito) para simulantes de alimentos. Ciência e Tecnologia de Alimentos 2004, 24, 397–402. [Google Scholar] [CrossRef]
- Drago, S.R. Chapter 5—Minerals. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Galanakis, C.M., Ed.; Academic Press: London, UK, 2016; pp. 129–157. ISBN 978-0-12-805257-0. [Google Scholar]
- Glahn, R. The use of Caco-2 cells in defining nutrient bioavailability: Application to iron bioavailability of foods. In Designing Functional Foods; McClements, D.J., Decker, E.A., Eds.; Elsevier: London, UK, 2009; pp. 340–361. [Google Scholar]
- Glahn, R.P.; Wortley, G.M.; South, P.K.; Miller, D.D. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: Studies using an In Vitro digestion/Caco-2 cell model. J. Agric. Food Chem. 2002, 50, 390–395. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value |
|---|---|
| Radiofrequency power | 1550 W |
| Argon flow rates | |
| Cooling | 13.8 L min−1 |
| Auxiliary | 0.65 L min−1 |
| Nebulizer | 1.05 L min−1 |
| Sample cone | Ni |
| Skimmer cone | Ni |
| Analyte | 43Ca, 56Fe, 65Cu, 66Zn |
| Internal standard | 103Rh (Fe, Cu, Zn), 45Sc (Ca, Fe), 89Y (Fe, Cu), 72Ge (Ca, Zn), 115In (Cu, Zn) |
| Aquisition/scanning mode | STD (Ca), KED (Fe, Cu, Zn) |
| Sweeps per reading | 100 |
| Dwell time | 10 ms (Ca, Cu, Zn); 40 ms (Fe) |
| No. of runs | 5 |
| Replicate time | 21 s |
| Sample uptake rate | 0.2 mL min−1 |
| Wash time between samples (2% HNO3) | 30 s |
| Sample delay | 50 s |
| Stabilization time | 5 s |
| Element | LOQ (µg kg−1) | Reference Material Measured Value (mg kg−1) | Reference Material Certificate Value (mg kg−1) |
|---|---|---|---|
| Ca | 29.8 | 0.66 ± 0.06 | 0.67 ± 0.04 |
| Fe | 1.8 | 306 ± 29 | 330 ± 20 |
| Cu | 7.2 | 8.4 ± 1.5 | 8.7 ± 0.5 |
| Zn | 6.6 | 34 ± 4 | 32 ± 2 |
| Household Processes | Iron (%) | Zinc (%) | Copper (%) |
|---|---|---|---|
| Regular pan with soaking water | 0.18 a | 33.94 a | 71.53 a |
| Pressure cooker with soaking water | 0.33 b | 44.66 b | 73.35 a |
| Regular pan without soaking water | 0.17 a | 31.55 a | 66.42 b |
| Pressure cooker without soaking water | 0.22 a | 35.04 a | 68.16 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feitosa, S.; Greiner, R.; Meinhardt, A.-K.; Müller, A.; Almeida, D.T.; Posten, C. Effect of Traditional Household Processes on Iron, Zinc and Copper Bioaccessibility in Black Bean (Phaseolus vulgaris L.). Foods 2018, 7, 123. https://doi.org/10.3390/foods7080123
Feitosa S, Greiner R, Meinhardt A-K, Müller A, Almeida DT, Posten C. Effect of Traditional Household Processes on Iron, Zinc and Copper Bioaccessibility in Black Bean (Phaseolus vulgaris L.). Foods. 2018; 7(8):123. https://doi.org/10.3390/foods7080123
Chicago/Turabian StyleFeitosa, Sabrina, Ralf Greiner, Ann-Katrin Meinhardt, Alexandra Müller, Deusdélia T. Almeida, and Clemens Posten. 2018. "Effect of Traditional Household Processes on Iron, Zinc and Copper Bioaccessibility in Black Bean (Phaseolus vulgaris L.)" Foods 7, no. 8: 123. https://doi.org/10.3390/foods7080123
APA StyleFeitosa, S., Greiner, R., Meinhardt, A. -K., Müller, A., Almeida, D. T., & Posten, C. (2018). Effect of Traditional Household Processes on Iron, Zinc and Copper Bioaccessibility in Black Bean (Phaseolus vulgaris L.). Foods, 7(8), 123. https://doi.org/10.3390/foods7080123