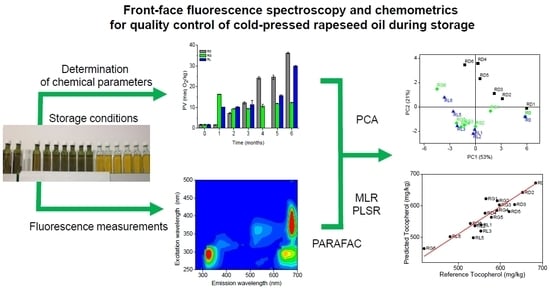
Front-Face Fluorescence Spectroscopy and Chemometrics for Quality Control of Cold-Pressed Rapeseed Oil During Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Storage Conditions
2.2. Determination of Chemical Parameters
2.3. Fluorescence Measurements
2.4. Data Analysis
3. Results and Discussion
3.1. Evolution of Chemical Parameters of Cold-Pressed Rapeseed Oil during Storage
3.2. Evolution of Fluorescence of Rapeseed Oil During Storage
3.3. Correlations between Chemical and Fluorescence Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matthäus, B.; Brühl, L. Why is it so difficult to produce high-quality virgin rapeseed oil for human consumption? Eur. J. Lipid Sci. Technol. 2008, 110, 611–617. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Quality of cold-pressed edible rapeseed oil in Germany. Nahrung 2003, 47, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Tynek, M.; Pawłowicz, R.; Gromadzka, J.; Tylingo, R.; Wardencki, W.; Karlovits, G. Virgin rapeseed oils obtained from different rape varieties by cold pressed method—Their characteristics, properties, and differences. Eur. J. Lipid Sci. Technol. 2012, 114, 357–366. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Huang, F.; Liu, C.; Wang, H. Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J. Food Compos. Anal. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. F 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Koski, A.; Pekkarinen, S.; Hopia, A.; Wähäla, K.; Heinonen, M. Processing of rapeseed oil: Effects on sinapic acid derivative content and oxidative stability. Eur. Food Res. Technol. 2003, 217, 110–114. [Google Scholar] [CrossRef]
- Koski, A.; Psomiadou, E.; Tsimidou, M.; Hopia, A.; Kefalas, P.; Wähälä, K.; Heinonen, M. Oxidative stability and minor constituents of virgin olive oil and cold-pressed rapeseed oil. Eur. Food Res. Technol. 2002, 214, 294–298. [Google Scholar] [CrossRef]
- Pawłowicz, R.; Gromadzka, J.; Tynek, M.; Tylingo, R.; Wardencki, W.; Karlovits, G. The influence of the UV irradiation on degradation of virgin rapeseed oils. Eur. J. Lipid Sci. Technol. 2013, 115, 648–658. [Google Scholar] [CrossRef]
- Roszkowska, B.; Tańska, M.; Czaplicki, S.; Konopka, I. Variation in the composition and oxidative stability of commercial rapeseed oils during their shelf life. Eur. J. Lipid Sci. Technol. 2015, 117, 673–683. [Google Scholar] [CrossRef]
- Rękas, A.; Ścibisz, I.; Siger, A.; Wroniak, M. The effect of microwave pretreatment of seeds on the stability and degradation kinetics of phenolic compounds in rapeseed oil during long-term storage. Food Chem. 2017, 222, 43–52. [Google Scholar] [CrossRef]
- Rękas, A.; Siger, A.; Wroniak, M. The effect of microwave pre-treatment of rapeseed on the degradation kinetics of lipophilic bioactive compounds of the oil during storage. Grasas y Aceites 2018, 69, e233. [Google Scholar] [CrossRef] [Green Version]
- Rękas, A.; Siger, A.; Wroniak, M.; Ścibisz, I. Phytochemicals and antioxidant activity degradation kinetics during long-term storage of rapeseed oil pressed from microwave-treated seeds. Eur. J. Lipid Sci. Technol. 2018, 120, 1700283. [Google Scholar] [CrossRef]
- Wroniak, M.; Rękas, A. Nutritional value of cold-pressed rapeseed oil during long term storage as influenced by the type of packaging material, exposure to light & oxygen and storage temperature. J. Food Sci. Technol. 2016, 53, 1338–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Xu, F.; Ji, S.; Ji, J.; Qin, F.; Ju, X.; Wang, L. Influence of photooxidation on the lipid profile of rapeseed oil using UHPLC-QTOF-MS and multivariate data analysis. Anal. Methods 2019, 11, 2903–2917. [Google Scholar] [CrossRef]
- Kanavouras, A.; Hernandez-Munoz, P.; Coutelieris, F.A. Packaging of olive oil: Quality issues and shelf life predictions. Food Rev. Int. 2006, 22, 381–404. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Kanavouras, A.; Kiritsakis, K. Chemical analysis, quality control and packaging issues of olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 628–638. [Google Scholar] [CrossRef]
- Cheikhousman, R.; Zude, M.; Bouveresse, D.J.-R.; Léger, C.L.; Rutledge, D.N.; Birlouez-Aragon, I. Fluorescence spectroscopy for monitoring deterioration of extra virgin olive oil during heating. Anal. Bioanal. Chem. 2005, 382, 1438–1443. [Google Scholar] [CrossRef]
- Poulli, K.I.; Chantzos, N.V.; Mousdis, G.A.; Georgiou, C.A. Synchronous fluorescence spectroscopy: Tool for monitoring thermally stressed edible oils. J. Agric. Food Chem. 2009, 57, 8194–8201. [Google Scholar] [CrossRef]
- Poulli, K.I.; Mousdis, G.A.; Georgiou, C.A. Monitoring olive oil oxidation under thermal and UV stress through synchronous fluorescence spectroscopy and classical assays. Food Chem. 2009, 117, 499–503. [Google Scholar] [CrossRef]
- Sikorska, E.; Khmelinskii, I.V.; Sikorski, M.; Caponio, F.; Bilancia, M.T.; Pasqualone, A.; Gomes, T. Fluorescence spectroscopy in monitoring of extra virgin olive oil during storage. Int. J. Food Sci. Technol. 2008, 43, 52–61. [Google Scholar] [CrossRef]
- Tena, N.; Garcia-Gonzalez, D.L.; Aparicio, R. Evaluation of virgin olive oil thermal deterioration by fluorescence spectroscopy. J. Agric. Food Chem. 2009, 57, 10505–10511. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Aparicio, R.; García-González, D.L. Chemical changes of thermoxidized virgin olive oil determined by excitation–emission fluorescence spectroscopy (EEFS). Food Res. Int. 2012, 45, 103–108. [Google Scholar] [CrossRef]
- Guzmán, E.; Baeten, V.; Pierna, J.A.F.; García-Mesa, J.A. Evaluation of the overall quality of olive oil using fluorescence spectroscopy. Food Chem. 2015, 173, 927–934. [Google Scholar] [CrossRef]
- Mbesse Kongbonga, Y.; Ghalila, H.; Majdi, Y.; Mbogning Feudjio, W.; Ben Lakhdar, Z. Investigation of heat-induced degradation of virgin olive oil using front face fluorescence spectroscopy and chemometric analysis. J. Am. Oil Chem. Soc. 2015, 92, 1399–1404. [Google Scholar] [CrossRef]
- Saleem, M.; Ahmad, N.; Ali, H.; Bilal, M.; Khan, S.; Ullah, R.; Ahmed, M.; Mahmood, S. Investigating temperature effects on extra virgin olive oil using fluorescence spectroscopy. Laser Phys. 2017, 27, 125602. [Google Scholar] [CrossRef]
- Domínguez Manzano, J.; Muñoz de la Peña, A.; Durán Merás, I. Front-face fluorescence combined with second-order multiway classification, based on polyphenol and chlorophyll compounds, for virgin olive oil monitoring under different photo- and thermal-oxidation procedures. Food Anal. Methods 2019, 12, 1399–1411. [Google Scholar] [CrossRef]
- Hakonen, A.; Beves, J.E. Hue Parameter fluorescence identification of edible oils with a smartphone. ACS Sens. 2018, 3, 2061–2065. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 3960:2007: Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- International Organization for Standardization. ISO 660:2009: Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. ISO 3656:2011: Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Association of Official Analytical Chemists International. Official Methods of Analysis of Association of Official Analytical Chemists International; AOAC Press: Arlington, VA, USA, 2003. [Google Scholar]
- Caponio, F.; Gomes, T.; Summo, C. Assessment of the oxidative and hydrolytic degradation of oils used as liquid medium of in-oil preserved vegetables. J. Food. Sci. 2003, 68, 147–151. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Sikorska, E. Simple reversed-phase liquid chromatography method for determination of tocopherols in edible plant oils. J. Chromatogr. A 2004, 1048, 195–198. [Google Scholar] [CrossRef]
- Zechmeister, L. Cis-Trans Isomeric Carotenoids, Vitamins A, and Arylpolyenes; Springer: Vienna, Austria, 1962. [Google Scholar]
- American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemistry Society; AOCS Press: Washington, DC, USA, 1993. [Google Scholar]
- Andersen, C.M.; Bro, R. Practical aspects of PARAFAC modeling of fluorescence excitation-emission data. J. Chemom. 2003, 17, 200–215. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Standard for Named Vegetable Oils; Codex Stan. 210; Codex Alimentarius Comission: Rome, Italy, 1999. [Google Scholar]
- Caponio, F.; Bilancia, M.; Pasqualone, A.; Sikorska, E.; Gomes, T. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur. Food Res. Technol. 2005, 221, 92–98. [Google Scholar] [CrossRef]
- Bilancia, M.T.; Caponio, F.; Sikorska, E.; Pasqualone, A.; Summo, C. Correlation of triacylglycerol oligopolymers and oxidised triacylglycerols to quality parameters in extra virgin olive oil during storage. Food Res. Int. 2007, 40, 855–861. [Google Scholar] [CrossRef]
- Gomes, T.; Caponio, F.; Durante, V.; Summo, C.; Paradiso, V.M. The amounts of oxidized and oligopolymeric triacylglycerols in refined olive oil as a function of crude oil oxidative level. LWT Food Sci. Technol. 2012, 45, 186–190. [Google Scholar] [CrossRef]
- Caponio, F.; Summo, C.; Bilancia, M.T.; Paradiso, V.M.; Sikorska, E.; Gomes, T. High performance size-exclusion chromatography analysis of polar compounds applied to refined, mild deodorized, extra virgin olive oils and their blends: An approach to their differentiation. LWT Food Sci. Technol. 2011, 44, 1726–1730. [Google Scholar] [CrossRef]
- Zandomeneghi, M.; Carbonaro, L.; Caffarata, C. Fluorescence of vegetable oils: Olive oils. J. Agric. Food Chem. 2005, 53, 759–766. [Google Scholar] [CrossRef]
- Guimet, F.; Ferré, J.; Boqué, R.; Rius, F.X. Application of unfold principal component analysis and parallel factor analysis to the exploratory analysis of olive oils by means of excitation–emission matrix fluorescence spectroscopy. Anal. Chim. Acta 2004, 515, 75–85. [Google Scholar] [CrossRef]







| Parameter | Time | Storage Conditions | Time*Storage Conditions |
|---|---|---|---|
| Oxygen in the bottle headspace | 0.378 | 0.014 | 0.707 |
| Oxygen in the oil | 0.210 | 0.616 | 0.081 |
| PV | <0.001 | 0.034 | <0.001 |
| K232 | 0.009 | 0.089 | 0.292 |
| K270 | 0.002 | 0.003 | 0.333 |
| Ox-TAG | 0.002 | 0.609 | 0.281 |
| TAGP | <0.001 | 0.039 | 0.181 |
| AV | 0.003 | 0.543 | 0.005 |
| DAG | 0.021 | 0.040 | 0.381 |
| Tocopherols | 0.006 | 0.022 | 0.577 |
| Carotenoids | <0.001 | 0.160 | 0.488 |
| Pheophytins | <0.001 | <0.001 | 0.105 |
| Parameter 1 | Regression Method | LV | R2cal | R2cv | RMSECV | RE (%) |
|---|---|---|---|---|---|---|
| K232 | MLR | 0.774 | 0.625 | 1.1 | 29 | |
| PLSR | 3 | 0.747 | 0.640 | 1.1 | 29 | |
| Ox-TAG (g/kg) | MLR | 0.732 | 0.393 | 3.9 | 22 | |
| PLSR | 2 | 0.678 | 0.498 | 3.7 | 21 | |
| TAGP (g/kg) | MLR | 0.853 | 0.754 | 0.061 | 18 | |
| PLSR | 3 | 0.853 | 0.799 | 0.059 | 17 | |
| Tocopherols (mg/kg) | MLR | 0.903 | 0.825 | 27.2 | 4.7 | |
| PLSR | 3 | 0.885 | 0.807 | 30.2 | 5.2 | |
| Carotenoids (mg/L) | MLR | 0.861 | 0.735 | 0.25 | 5.4 | |
| PLSR | 2 | 0.836 | 0.765 | 0.25 | 5.4 | |
| Pheophytins (%) | MLR | 0.984 | 0.965 | 7.03 | 13.7 | |
| PLSR | 3 | 0.984 | 0.972 | 6.65 | 12.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska, E.; Wójcicki, K.; Kozak, W.; Gliszczyńska-Świgło, A.; Khmelinskii, I.; Górecki, T.; Caponio, F.; Paradiso, V.M.; Summo, C.; Pasqualone, A. Front-Face Fluorescence Spectroscopy and Chemometrics for Quality Control of Cold-Pressed Rapeseed Oil During Storage. Foods 2019, 8, 665. https://doi.org/10.3390/foods8120665
Sikorska E, Wójcicki K, Kozak W, Gliszczyńska-Świgło A, Khmelinskii I, Górecki T, Caponio F, Paradiso VM, Summo C, Pasqualone A. Front-Face Fluorescence Spectroscopy and Chemometrics for Quality Control of Cold-Pressed Rapeseed Oil During Storage. Foods. 2019; 8(12):665. https://doi.org/10.3390/foods8120665
Chicago/Turabian StyleSikorska, Ewa, Krzysztof Wójcicki, Wojciech Kozak, Anna Gliszczyńska-Świgło, Igor Khmelinskii, Tomasz Górecki, Francesco Caponio, Vito M. Paradiso, Carmine Summo, and Antonella Pasqualone. 2019. "Front-Face Fluorescence Spectroscopy and Chemometrics for Quality Control of Cold-Pressed Rapeseed Oil During Storage" Foods 8, no. 12: 665. https://doi.org/10.3390/foods8120665
APA StyleSikorska, E., Wójcicki, K., Kozak, W., Gliszczyńska-Świgło, A., Khmelinskii, I., Górecki, T., Caponio, F., Paradiso, V. M., Summo, C., & Pasqualone, A. (2019). Front-Face Fluorescence Spectroscopy and Chemometrics for Quality Control of Cold-Pressed Rapeseed Oil During Storage. Foods, 8(12), 665. https://doi.org/10.3390/foods8120665